Composition, synthesis and application of hydantoin derivatives
A technology of hydantoin and derivatives, which is applied in the composition of hydantoin derivatives and its medical application, and can solve the problems that the research and development of prostate cancer drugs cannot be carried out accurately.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
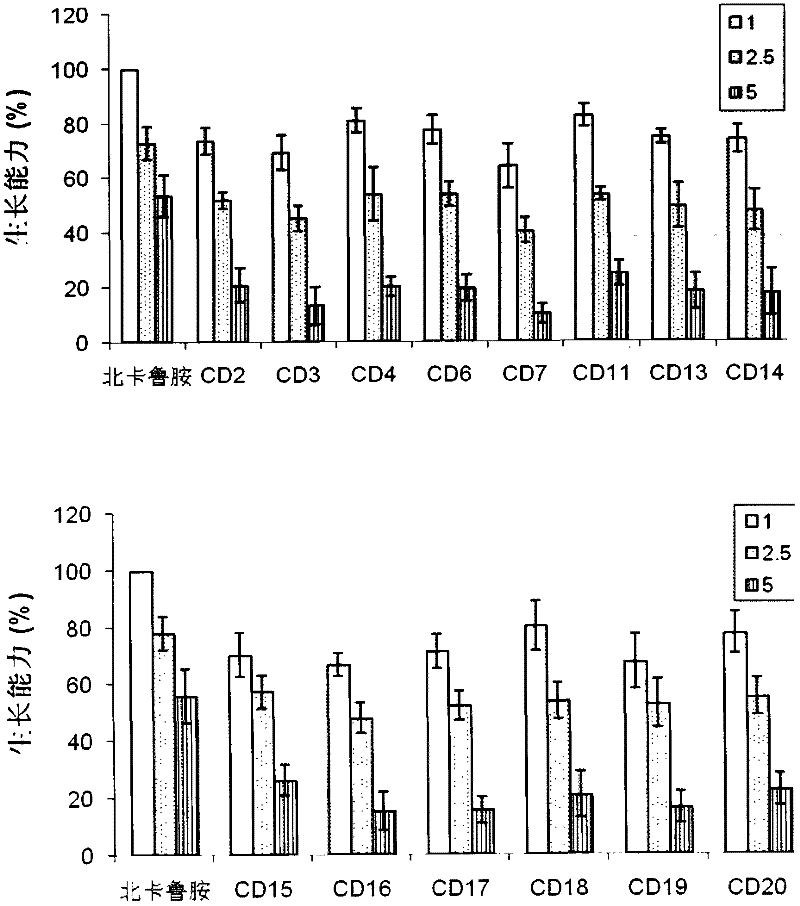
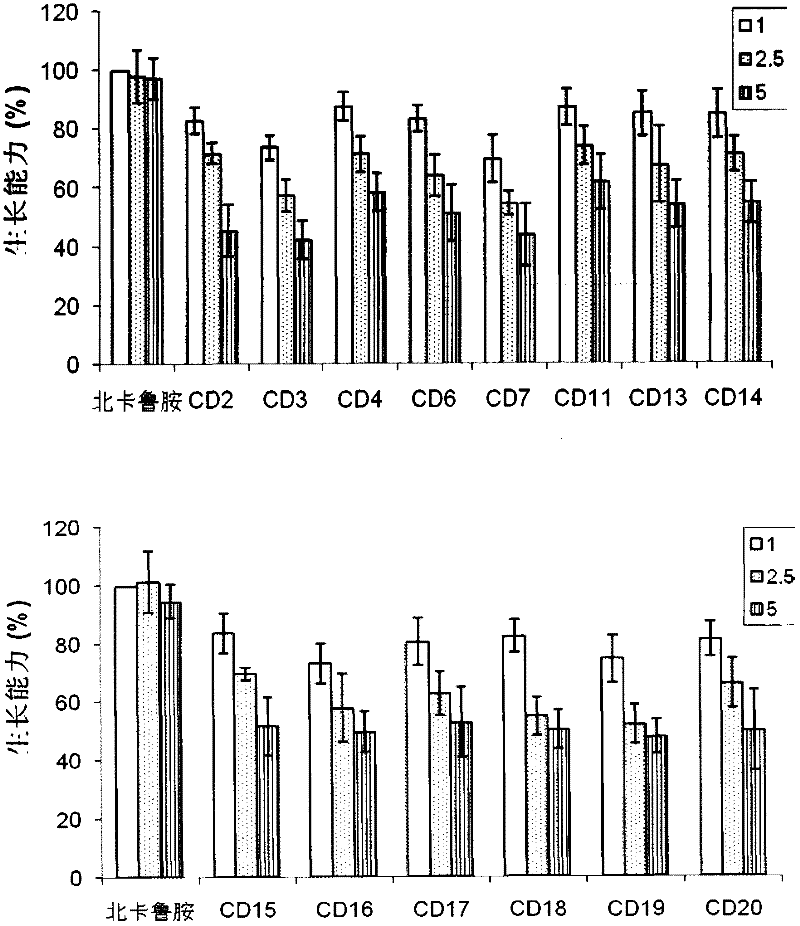
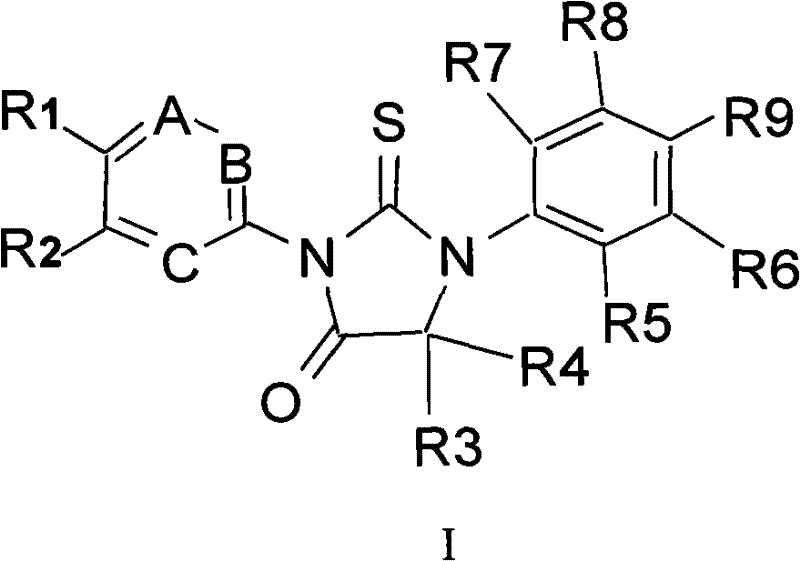
Image
Examples
Embodiment Construction
[0052] Compound CD1
[0053] 4-[3-(6-cyano-5-trifluoromethylpyridin-3-yl)-5,5-dimethyl-4-oxo-2-thioimidazolidin-1-yl]-benzene Butanamide
[0054]
[0055] 5-Amino-2-cyano-3-(trifluoromethyl)pyridine (10 g) dissolved in 18 mL THF was slowly added to an aqueous solution (120 mL) containing 5 mL thiophosgene at room temperature After stirring for 50 minutes, it was extracted with dichloromethane (100 ml three times), the organic phase was washed with salt, and washed with anhydrous Na 2 SO 4 Drying, concentration under reduced pressure, passing through a silica gel column, and eluting with PE-EA (20:1) gave white solid compound 1A (5-isothiocyanate-2-cyano-3-(trifluoromethyl)pyridine, 10 gram).
[0056] Combine p-aminophenylbutyric acid (2 g), acetone cyanohydrin (16 ml) and MgSO 4 (6g) was heated to 80°C, stirred for 24 hours, added 50 ml of ethyl acetate and water for extraction after room temperature, and the organic phase was passed over MgSO 4 , concentrated under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com