Anode and cathode conductive additive for secondary lithium battery, method for preparing conductive additive, and method for preparing secondary lithium battery
A conductive additive, lithium secondary battery technology, applied in battery electrodes, electrolyte battery manufacturing, non-aqueous electrolyte battery and other directions, can solve the problems of limited practical application, high price, difficult to meet the continuous charging and discharging of batteries, etc., to achieve high rate Charge and discharge, improve high temperature performance, the effect of superior performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
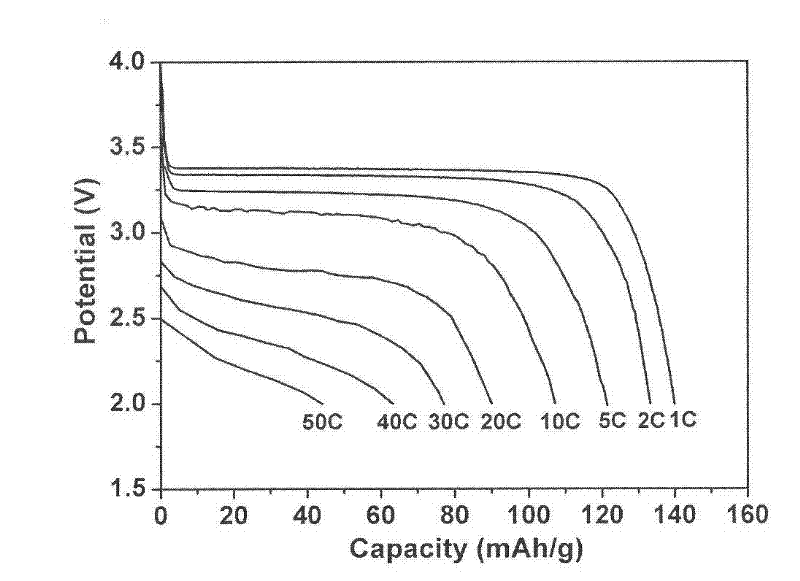
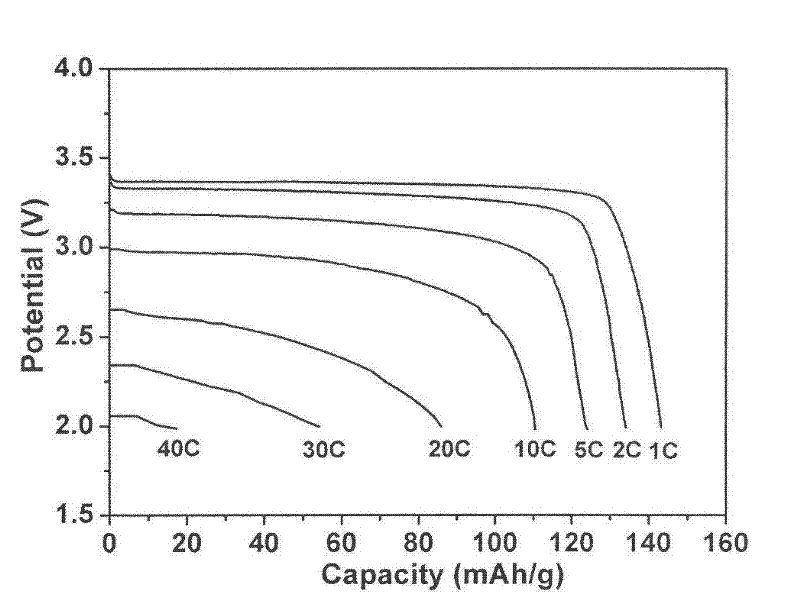
[0042] Weigh 0.2g of polyvinylidene fluoride (PVDF) binder and dissolve it in 5 grams of N-methylpyrrolidone, then add 0.6g of graphene powder conductive additive, and stir evenly. Subsequently, 3.2 g of lithium iron phosphate positive electrode active material was added, stirred and dispersed for 1 hour. The obtained slurry was uniformly coated on an aluminum foil, dried under vacuum at 100° C., and rolled to form a positive electrode sheet. with LiPF 6 The solution is an electrolyte, and Cellgard2400 is used as a diaphragm to assemble a lithium-ion battery. The measured discharge capacity was 142mAh / g at 1C, 110mAh / g at 10C, and 80mAh / g at 30C. After the battery is charged and discharged 500 times at 1C, the capacity decay is less than 5%.
Embodiment 2
[0044] Weigh 0.2g of polyvinylidene fluoride (PVDF) binder and dissolve it in 5g of N-methylpyrrolidone, then add 0.2g of graphene powder conductive additive, and stir evenly. Subsequently, 3.6 g of lithium iron phosphate positive electrode active material was added, stirred and dispersed for 1 hour. The obtained slurry was uniformly coated on an aluminum foil, dried under vacuum at 100° C., and rolled to form a positive electrode sheet. with LiPF 6 The solution is an electrolyte, and Cellgard2400 is used as a diaphragm to assemble a lithium-ion battery. The measured discharge capacity was 140mAh / g at 1C, 100mAh / g at 10C, and 60mAh / g at 30C. After the battery is charged and discharged 500 times at 1C, the capacity decay is less than 5%.
Embodiment 3
[0046] Weighing 1g of graphene solids was added to 100g of N-methylpyrrolidone, and ultrasonically treated for 15 minutes to obtain a uniformly dispersed graphene sol. The above-mentioned graphene sol was concentrated to a slurry with a graphene mass concentration of 5% by centrifugation.
[0047] Weigh 0.2g of polyvinylidene fluoride (PVDF) binder and dissolve it in 1 gram of N-methylpyrrolidone, then add 4g of graphene slurry with a mass concentration of 5% prepared in the previous step, and stir evenly. Subsequently, 3.6 g of lithium iron phosphate positive electrode active material was added, stirred and dispersed for 1 hour. The obtained slurry was uniformly coated on an aluminum foil, dried under vacuum at 100° C., and rolled to form a positive electrode sheet. with LiPF 6 The solution is an electrolyte, and Cellgard2400 is used as a diaphragm to assemble a lithium-ion battery. The measured discharge capacity was 140mAh / g at 1C, 105mAh / g at 10C, and 69mAh / g at 30C. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com