Sheet-form preparation and method for producing the same
A preparation and tablet technology, applied in the field of gel-type tablet preparations, can solve problems such as difficulty in maintaining the shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
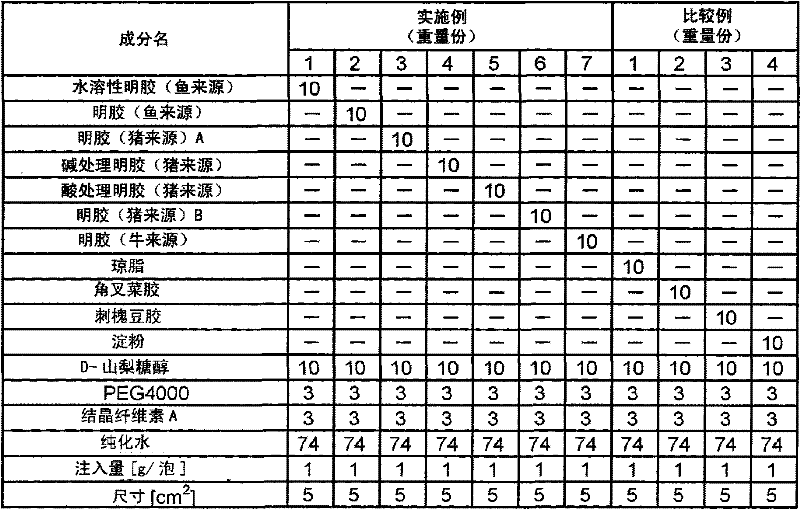
[0105] 3 parts by weight of crystalline cellulose A (average particle diameter: 20 μm) was added to 74 parts by weight of purified water, and ultrasonic dissolution and dispersion were performed. Add 10 parts by weight of water-soluble gelatin (about 100,000 average molecular weight, about 8.6 mol% of hydroxyproline) derived from fish (tilapia) therein, dissolve it at a temperature of normal temperature to 40° C., and further add Add 10 parts by weight of D-sorbitol, 3 parts by weight of PEG4000 to make it dissolve, and distribute 1g to 5cm 2 The plastic blister (Cryomold (square) No. 3, manufactured by Sakura Finetek Japan Co., Ltd.) was cooled and solidified at 2 to 8°C for 1 day and night to obtain a sheet-like preparation.
Embodiment 2
[0107] 3 parts by weight of crystalline cellulose A (average particle diameter: 20 μm) was added to 74 parts by weight of purified water, and ultrasonic dissolution and dispersion were performed. 10 parts by weight of gelatin (derived from fish) (average molecular weight: 100,000, hydroxyproline content about 8.6 mol%) was added thereto, dissolved at a temperature of 50 to 70°C, and vibrated at a constant temperature of 40°C. In this solution, add 10 parts by weight of D-sorbitol, 3 parts by weight of PEG4000 to make it dissolve, and distribute 1g to 5cm 2 The plastic blister (Cryomold (square) No. 3, manufactured by Sakura Finetek Japan Co., Ltd.) was cooled and solidified at 2 to 8°C for 1 day and night to obtain a sheet-like preparation.
Embodiment 3~7
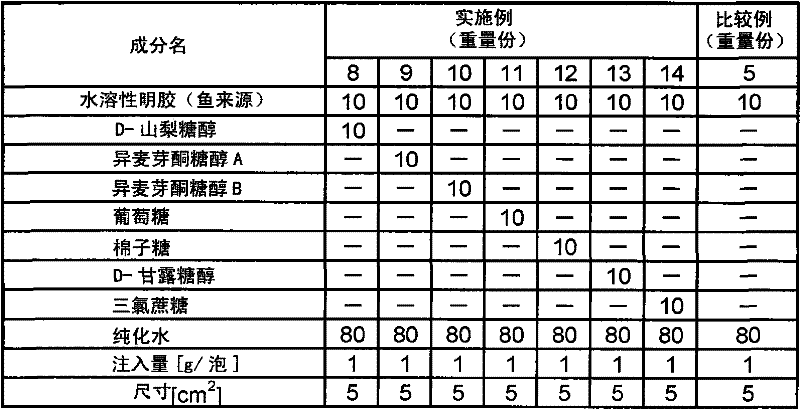
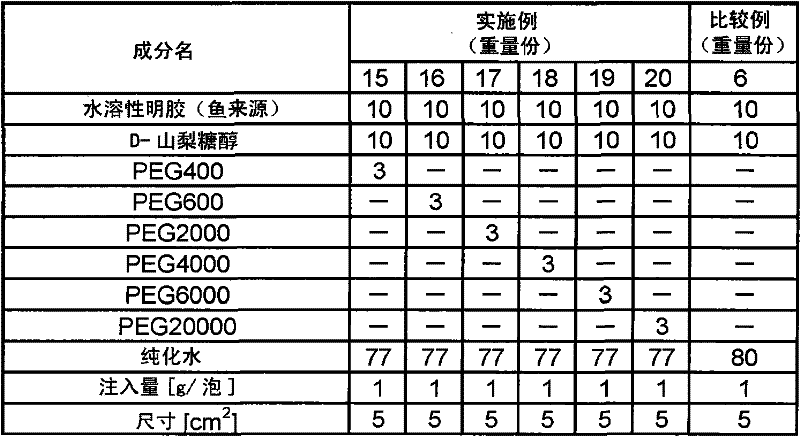
[0109] Except having adopted the composition shown in Table 1, it operated in the same procedure as Example 2, and obtained the sheet-form preparation.
[0110] Use gelatin (pig origin) A (average molecular weight about 85,000, hydroxyproline amount about 9.2 mole %) in embodiment 3, use alkali treatment gelatin (pig origin) (average molecular weight 180,000, hydroxyproline amount) in embodiment 4 Acid amount is about 9.2 mol%), acid-treated gelatin (porcine origin) is used in Example 5 (average molecular weight 100,000, hydroxyproline amount is about 9.2 mol%), and gelatin (porcine origin) B (average Molecular weight about 100,000, hydroxyproline content about 9.4 mol%), gelatin (bovine origin) was used in Example 7 (average molecular weight about 200,000, hydroxyproline content about 9.5 mol%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com