Film-form preparation
A thin-film and preparation technology, which is applied in the direction of bulk delivery, sheet delivery, sugar derivative preparation, etc., can solve the problems of frequent hospital visits, pain, and difficulty in controlling oral dissolution time arbitrarily
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
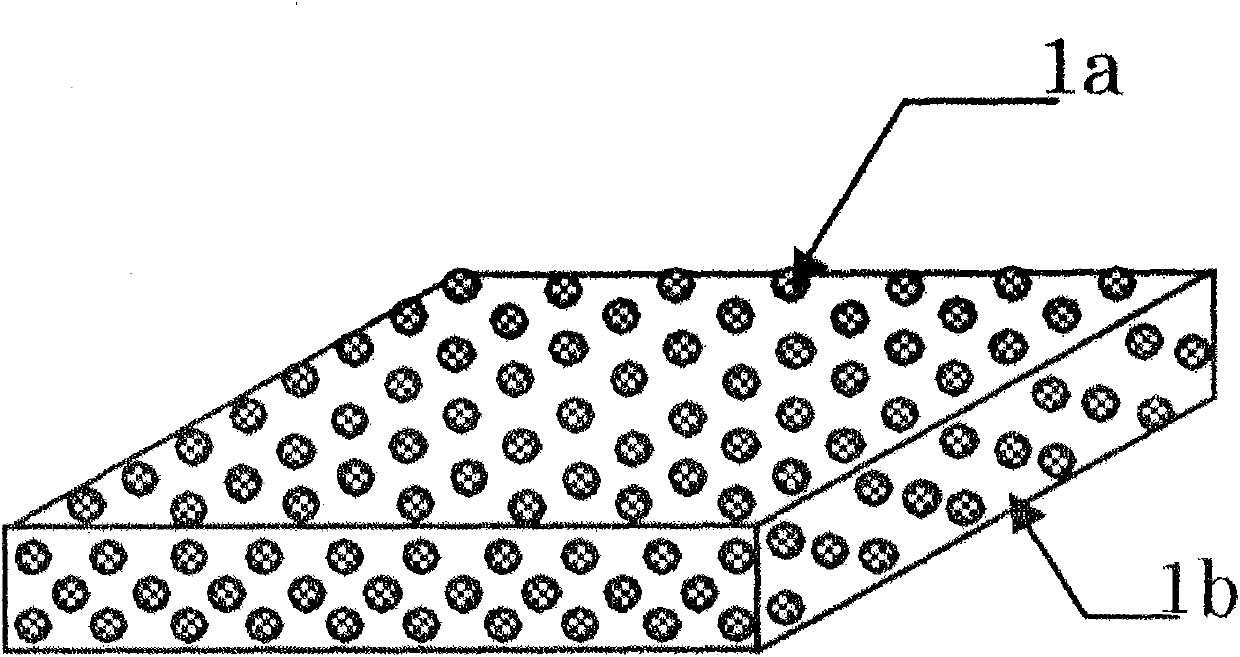
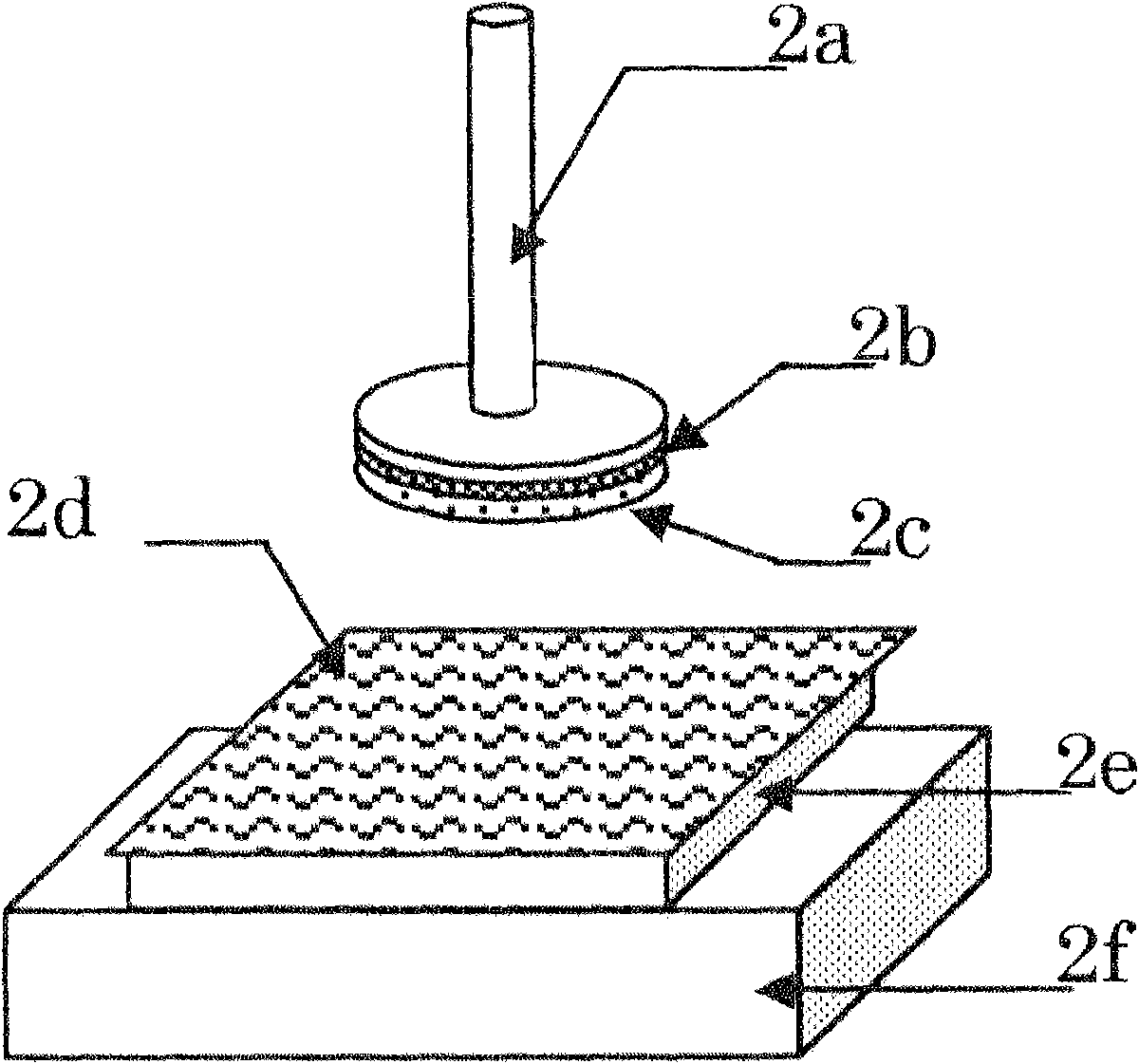
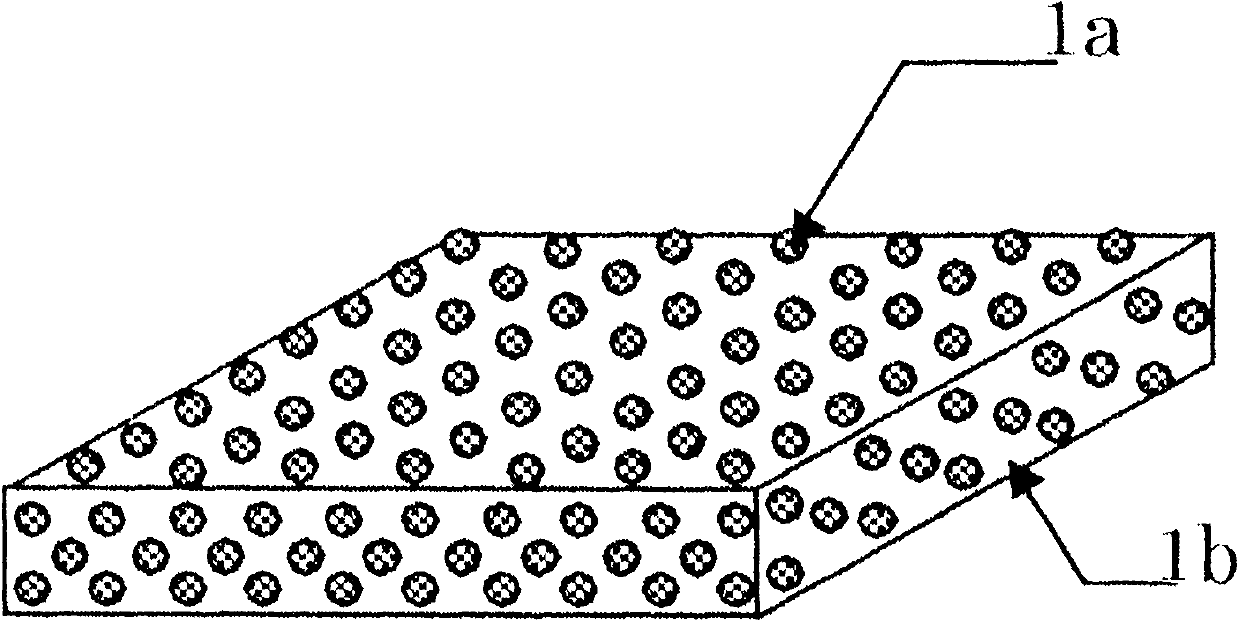
[0098] 67.5 parts by weight of D-mannitol microparticles whose particle size was previously controlled, and 0.8 parts by weight of polyethylene glycol were added to 150.0 parts by weight of ethanol, and ultrasonically dispersed. 64.5 parts by weight of HP C (HP C-SSL, manufactured by Nippon Soda) with a weight average molecular weight of about 30,000 and a hydroxypropoxyl substitution degree of 53.4 to 77.5% was added to the obtained substance, and stirred and dissolved. Further, 30.0 parts by weight of a standardized allergen treatment extract "TO RII" cedar pollen 2000 JAU / mL (manufactured by Torii Pharmaceutical Co., Ltd.) was added, and stirred and mixed with a rolling mixer to prepare a particle dispersion.
[0099] After the particle dispersion was sufficiently degassed, it was developed and dried on a polyester release film to produce a film with a thickness of about 100 μm. Cut the obtained film into 4cm 2 Rectangular, to obtain film-like preparations.
Embodiment 2
[0101] A film-form preparation was obtained in the same manner as in Example 1 except that acetone was used instead of ethanol.
Embodiment 3
[0103] A film-form preparation was obtained in the same manner as in Example 1 except that the composition shown in Table 2 was used.
[0104] In addition, "PVP K-30" in Table 2 is polyvinylpyrrolidone (manufactured by Nippon Shokubai Co., Ltd.) with a weight average molecular weight of about 40,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com