Production process of unstable chemical injection
A technology of stabilizers and substances, applied in the production process of unstable compound injections, which can solve problems such as product precipitation, turbidity, and decomposition of adenosine triphosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
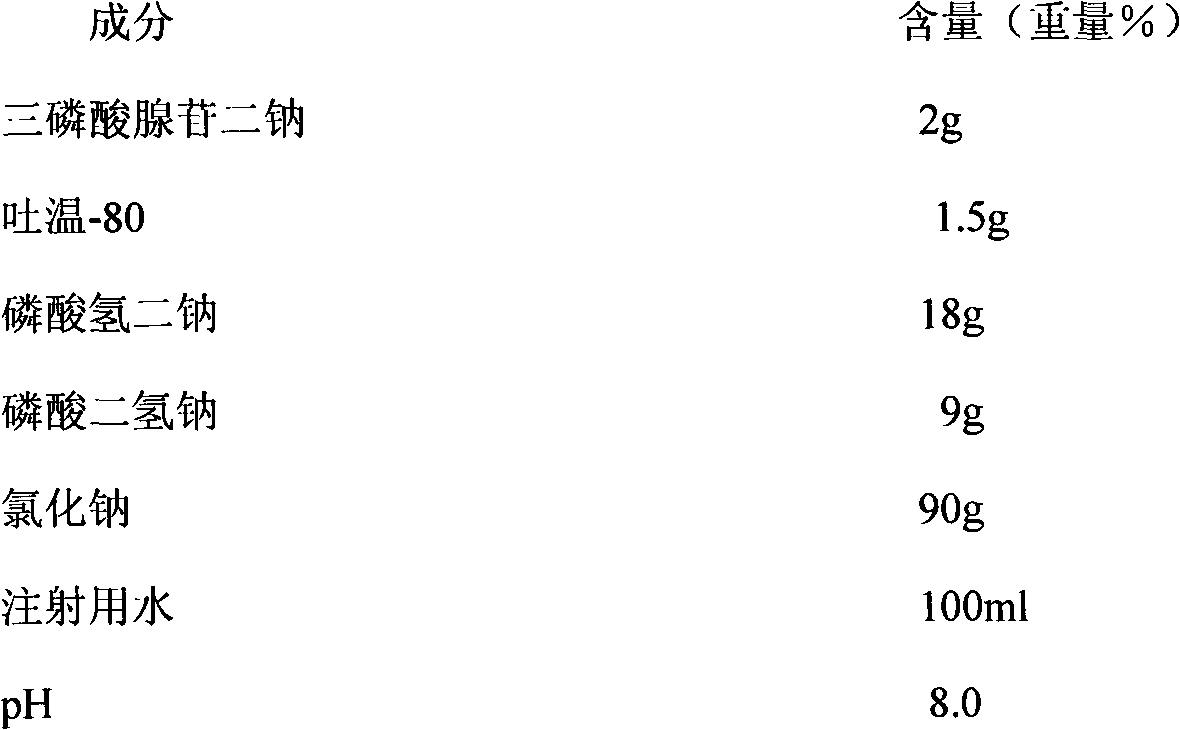
[0017] Preparation Example 1: Injection stock solution containing adenosine triphosphate disodium sodium chloride:
[0018]
[0019]
[0020] Take by weighing a certain amount of guanidine carbonate and dissolve it in an appropriate amount of water for injection, add disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, adenosine triphosphate disodium successively and fully stir to dissolve, add 0.05% (mg / ml) weight of Use activated carbon for needles, stir thoroughly, decarbonize by coarse filtration, and add water to 100ml. Sterilize with circulating steam at 120°C for 30 minutes. Cool, dilute to 1000ml with sterile water for injection, filter through a 0.22μm membrane filter, pressurize the filtrate for 20min under a pressure of 300MPa, pack it in a can, and seal it with a sterilized aluminum cap; pack it to obtain adenosine triphosphate disodium chloride injection liquid finished product.
preparation example 2
[0021] Preparation Example 2: Injection stock solution containing adenosine triphosphate disodium sodium chloride:
[0022]
[0023] Weigh a certain amount of Tween-80 and dissolve it in an appropriate amount of water for injection, add disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, and adenosine triphosphate disodium in sequence to dissolve, and add 0.05% (mg / ml) by volume Activated carbon was used for the weight of the needle, and after stirring thoroughly, the coarse filter was used to decarbonize, and water was added to 100ml. Sterilize with circulating steam at 120°C for 30 minutes. Cool, dilute to 1000ml with sterile water for injection, filter through a 0.22μm membrane filter, pressurize the filtrate for 20min under a pressure of 300MPa, pack it in a can, and seal it with a sterilized aluminum cap; pack it to obtain adenosine triphosphate disodium chloride injection liquid finished product.
preparation example 3
[0024] Preparation example 3: the stabilizer in embodiment 1 or 2 is replaced with the same content of propylene glycol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com