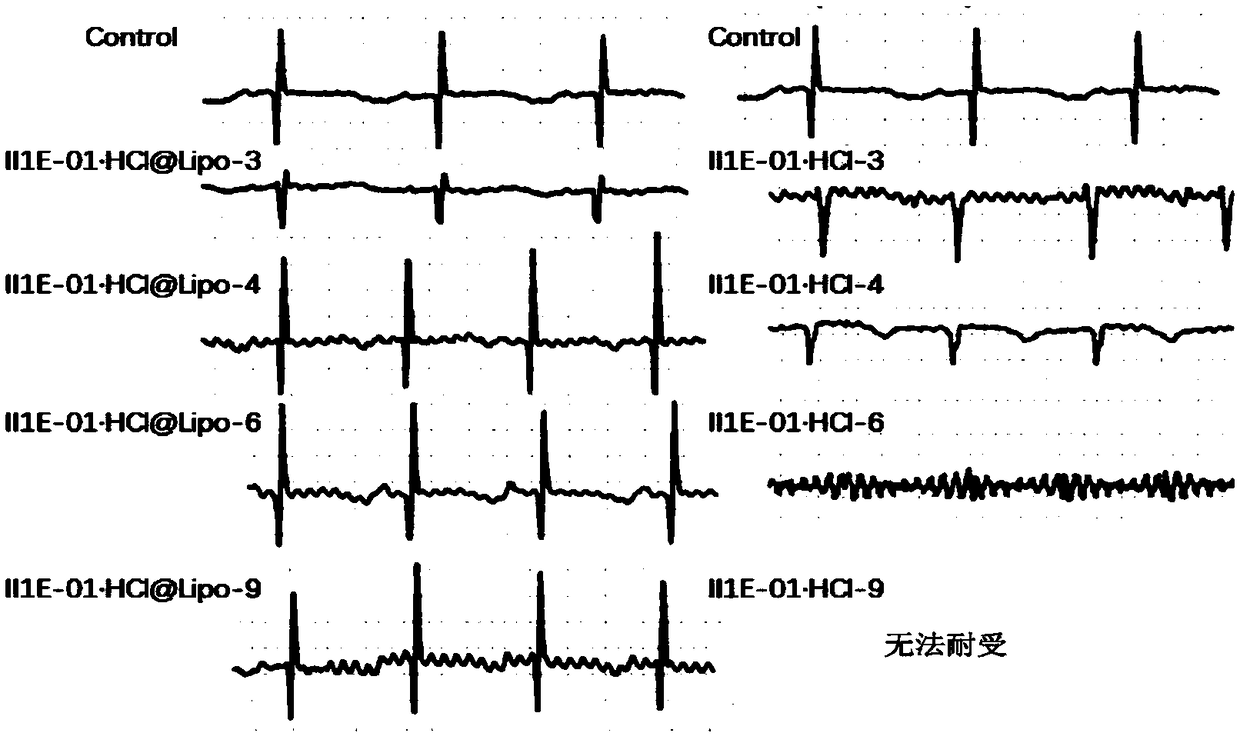
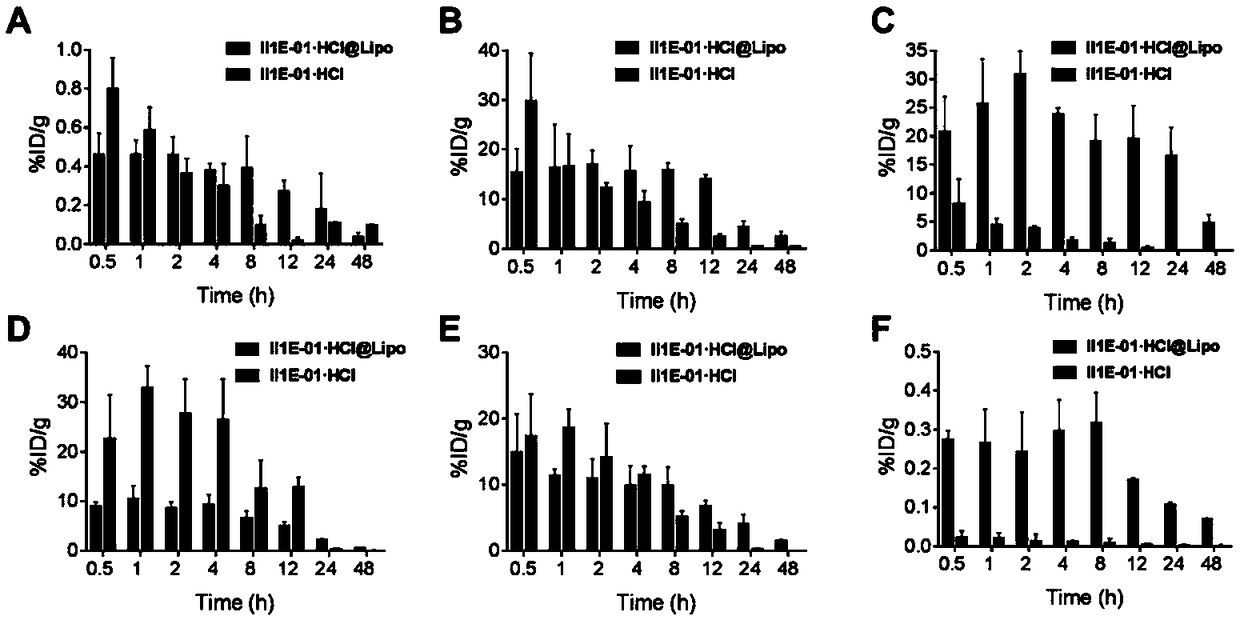
Cardiac glycoside active compound lipidosome and preparation method thereof
A compound, cardiac glycoside technology, applied in the field of cardiac glycoside active compound liposome and its preparation, can solve problems such as cardiac toxicity and side effects, achieve the effects of reducing toxicity and side effects, expanding the scope of application, and increasing distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1II1E-01
[0052] Example 1 Preparation of II1E-01·HCl liposome (II1E-01·HCl@Lipo)
[0053] The hydrogenated soybean lecithin, cholesterol, and distearoylphosphatidylethanolamine-polyethylene glycol copolymer were mixed in a weight ratio of (56:20:24), dissolved in absolute ethanol, and placed on a rotary evaporator at 40 Rotary evaporation at -60℃ and pressure of 0.02-5.32kPa to remove the absolute ethanol and form a lipid film; then add 0.11M ammonium sulfate aqueous solution at the same temperature for hydration, and continue to rotate for 0.5h under normal pressure ; Use ultrasound (30% power, 10min) to uniform the particle size of the liposome suspension formed, and use column chromatography to replace the external phase with deionized water to form an ammonium sulfate gradient inside and outside the phospholipid membrane, which is a blank Liposomes. The II1E-01·HCl solution (according to the mass ratio of the drug to the membrane material is 8:100) and the blank liposome are respecti...
Embodiment 2C01
[0054] Example 2 Preparation of C01 liposome (C01@Lipo)
[0055] Mix the hydrogenated soybean lecithin, cholesterol and distearoyl phosphatidylethanolamine-polyethylene glycol copolymer in a mass ratio of (56:20:24), and add C01 (according to the mass percentage of the drug and the membrane material is 8:100) , Completely dissolved in anhydrous ethanol and heated at 50℃, slowly poured into PBS (pH 8.0) stirred at the same temperature, after the injection is completed, continue to open the heating and stirring to volatilize the anhydrous ethanol; finally mix the formed liposomes The suspension is ultrasonic (30% power, 10min) with uniform particle size and passed through a 0.22μm microporous filter membrane to remove the drugs not encapsulated in the liposomes, thereby obtaining C01@Lipo.
Embodiment 3
[0056] Example 3 Preparation of Bufalin Liposome (Bufalin@Lipo)
[0057] Bufalin was used to replace C01 in Example 2, and the remaining operations were the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com