Natamycin cationic liposome eye drop and preparation method thereof
A cationic liposome and natamycin technology, which is applied in liposome delivery, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of eye discomfort in patients with increased preparation costs, complex preparation process, and drug Large dosage and other problems, to achieve the effects of reducing drug leakage, good permeability, and high transcorneal transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
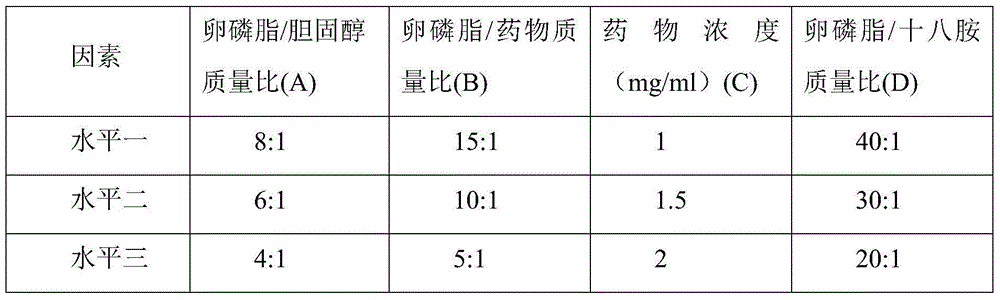
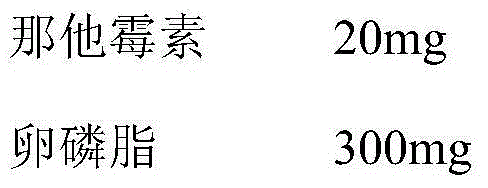
[0027] A kind of natamycin cationic liposome eye drop, comprises natamycin and phosphate buffer saline, and described eye drop also comprises lecithin, cholesterol and octadecylamine, and concrete component quantity is as follows:
[0028]
[0029]
[0030] The preparation technology of above-mentioned natamycin cationic liposome eye drops, comprises the steps:
[0031] Step 1: Dissolve natamycin in an appropriate amount of methanol, lecithin, cholesterol and octadecylamine in an appropriate amount of chloroform, mix them in a water bath at 48°C, rotate at 110 rpm, and evaporate at a reduced pressure of 0.08-0.09 MPa until a thin film is formed;
[0032] Step 2: Add PBS pH=7.0, 40°C water bath, rotate 140rpm for elution;
[0033] Step 3: Stir the obtained suspension magnetically for 30 minutes, and sonicate in an ultrasonic cleaner for 10 minutes to obtain the natamycin cationic liposome suspension.
Embodiment 2
[0035] A kind of natamycin cationic liposome eye drop, comprises natamycin and phosphate buffer saline, and described eye drop also comprises lecithin, cholesterol and octadecylamine, and concrete component quantity is as follows:
[0036]
[0037] The preparation technology of above-mentioned natamycin cationic liposome eye drops, comprises the steps:
[0038] Step 1: Dissolve natamycin in an appropriate amount of methanol, lecithin, cholesterol and octadecylamine in an appropriate amount of chloroform, mix them in a water bath at 48°C, rotate at 110 rpm, and evaporate at a reduced pressure of 0.08-0.09 MPa until a thin film is formed;
[0039] Step 2: Add PBS pH=7.0, 40°C water bath, rotate 140rpm for elution;
[0040] Step 3: Stir the obtained suspension magnetically for 30 minutes, and sonicate in an ultrasonic cleaner for 10 minutes to obtain the natamycin cationic liposome suspension.
Embodiment 3
[0042] A kind of natamycin cationic liposome eye drop, comprises natamycin and phosphate buffer saline, and described eye drop also comprises lecithin, cholesterol and octadecylamine, and concrete component quantity is as follows:
[0043]
[0044]
[0045] The preparation technology of above-mentioned natamycin cationic liposome eye drops, comprises the steps:
[0046] Step 1: Dissolve natamycin in an appropriate amount of methanol, lecithin, cholesterol and octadecylamine in an appropriate amount of chloroform, mix them in a water bath at 48°C, rotate at 110 rpm, and evaporate at a reduced pressure of 0.08-0.09 MPa until a thin film is formed;
[0047] Step 2: Add PBS pH=7.0, 40°C water bath, rotate 140rpm for elution;
[0048]Step 3: Stir the obtained suspension magnetically for 30 minutes, and sonicate in an ultrasonic cleaner for 10 minutes to obtain the natamycin cationic liposome suspension.
[0049] In order to further evaluate the quality and therapeutic benefi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com