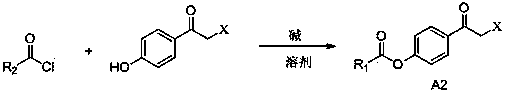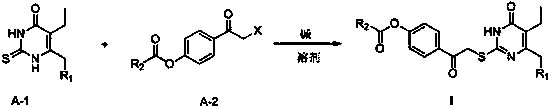2-(4-alkylformyloxyphenylcarbonylmethylthio)pyrimidine compounds and application thereof
A technology of hydrocarbyl formyloxybenzene and carbonylmethylthio, which is applied in the field of 2-pyrimidinone compounds to achieve good anti-HIV-1 virus activity, small cytotoxicity, and high selectivity index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
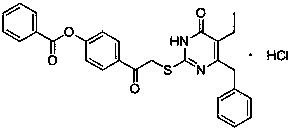
[0025] Example 1 : 5-ethyl-6-cyclohexylmethyl-2-[(4-benzoyloxy)phenylcarbonylmethylthio]pyrimidine-4(3 H )-ketone synthesis, concrete operation is as follows:
[0026] Dissolve 3 mmol of 5-ethyl-6-(cyclohexylmethyl)-2-thiouracil in 10 ml of dry N,N -Dimethylformamide solution, add K 2 CO 3 (3 mmol), stirred at room temperature for 0.5 hours, added α-bromo-4-benzoyloxyacetophenone (3.3 mmol), continued to stir at 40°C, TLC traced the disappearance of the raw material point for about 18 hours, and stopped the reaction , pour the reaction solution into 50 ml of ice water, stir to precipitate the precipitate, filter, wash the precipitate with water, suction filter and dry at 70°C to obtain the crude product, which is subjected to column chromatography (petroleum ether: ethyl acetate = 2:1) , to obtain white crystal 1a, the detection results are as follows:
[0027] 1a
[0028] Yield: 75%: Melting point: 169-170°C; 1 H NMR ((300MHz, CDCl 3 ) δ ppm 0.86-1.26 (m, 8H, C...
Embodiment 2
[0029] Example 2 : 5-ethyl-6-cyclohexylmethyl-2-[(4-cyclohexyloxy)phenylcarbonylmethylthio]pyrimidine-4(3 H )-ketone synthesis, concrete operation is as follows:
[0030] Dissolve 5-ethyl-6-(cyclohexylmethyl)-2-thiouracil (3 mmol) in 10 ml of dry N , N -Dimethylformamide solution, add K 2 CO 3 (3 mmol), stirred at room temperature for 0.5 hours, added α-chloro-4-cyclohexyloxyacetophenone (3.3 mmol), continued stirring reaction at 80 ° C, TLC traced the disappearance of the raw material point for about 20 hours, stopped For reaction, pour the reaction solution into 50 ml of ice water, stir to precipitate the precipitate, filter, wash the precipitate with water, suction filter and dry at 70°C to obtain the crude product, which is subjected to column chromatography (petroleum ether: ethyl acetate = 2:1) Finally, white crystal 1b was obtained, and the detection results were as follows:
[0031] 1b
[0032] Yield: 62%: Melting point: 154-155°C; 1 H NMR (300MHz, CDCl 3...
Embodiment 3
[0033] Example 3 : 5-ethyl-6-benzyl-2-[(4-benzoyloxy)phenylcarbonylmethylthio]pyrimidine-4(3 H )-ketone synthesis, concrete operation is as follows:
[0034] Dissolve 5-ethyl-6-benzyl-2-thiouracil (3 mmol) in 10 ml of dry N , N -Dimethylformamide solution, add K 2 CO 3 (3 mmol), stirred and reacted at room temperature for 0.5 hours, added α-bromo-4-benzoyloxyacetophenone (3.3 mmol), continued to stir and reacted at 20°C, TLC traced the disappearance of the raw material point for about 8 hours, and stopped the reaction , pour the reaction solution into 50 ml of ice water, stir to precipitate the precipitate, filter, wash the precipitate with water, suction filter and dry at 70°C to obtain the crude product, which is subjected to column chromatography (petroleum ether: ethyl acetate = 2:1) , to obtain white crystal 1c, the detection results are as follows:
[0035] 1c
[0036] Yield: 68%: Melting point: 172.6-173.8°C; 1 H NMR (300MHz, CDCl 3 ) δ ppm 1.04 (t, 3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com