Method for extracting indium and zinc from high-iron indium-containing zinc calcine and preparing iron oxide
An iron indium zinc and iron oxide technology, which is applied in the fields of extracting indium zinc and preparing iron oxide, can solve the problems that iron cannot be used as a resource, and the recovery rate of indium is low, and achieves easy resource utilization, improved leaching performance, and iron The effect of high slag grade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
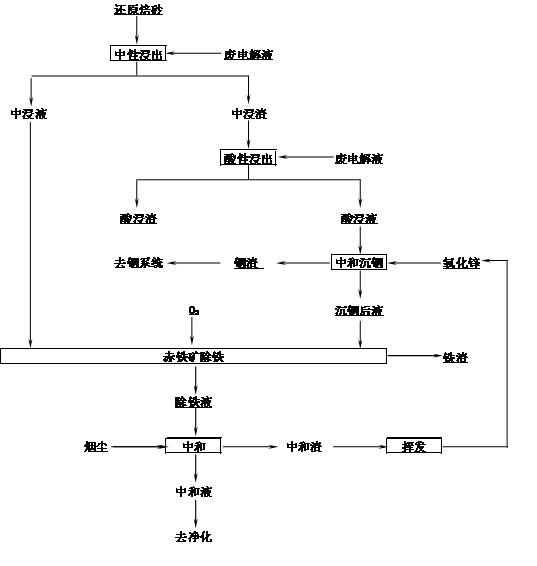
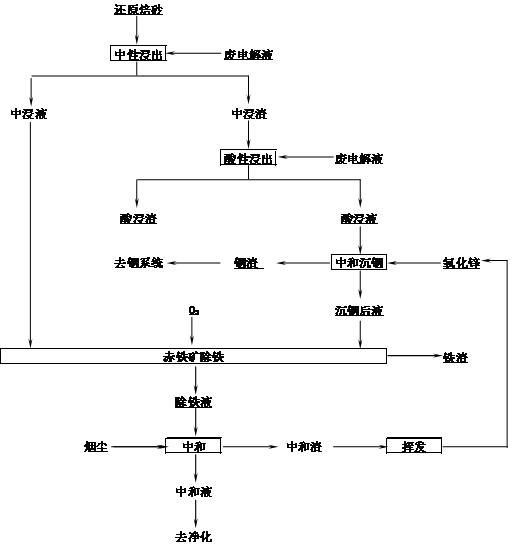
[0026] The method for extracting indium zinc and preparing iron oxide from high-iron indium zinc calcine of the present invention is carried out according to the following steps:
[0027] 1. The high-iron zinc calcined sand containing Zn 53.54%, Fe 19.42%, and In 0.092% is reduced in contact with CO in a rotary kiln, the reduction temperature is 870-900°C, and nitrogen gas containing 20% carbon monoxide is introduced, and the excess coefficient is 1.2. Add high-iron indium-zinc calcine at a rate of 6kg / h, install a lifting plate in the kiln, and rotate the material in the kiln for 30 minutes to discharge, and the material is lowered to room temperature in the same reducing atmosphere to obtain Fe 2+ 14.96% reduced calcine, its main chemical composition is:
[0028] Zn% Fe% Fe 2+ % In% Reduction rate% Reduced calcine 53.54 19.42 14.96 0.092 77.03
[0029] [0022] 2. The obtained reduced calcined sand is neutrally leached with sulfuric ...
Embodiment 2
[0036] A method for extracting indium zinc and preparing iron oxide from high-iron indium-zinc calcine, comprising the following steps:
[0037] 1. The high-iron indium-zinc calcine containing Zn50.9%, Fe20.81%, In0.10% is reduced in contact with CO in a rotary kiln, and nitrogen gas containing 10% carbon monoxide is introduced, the excess coefficient is 0.8, and the reduction temperature is 770 ~800℃, reduction time 80min, get Fe-containing 2+ 13.88% reduced calcine, the reduction rate is 66.7%.
[0038] 2. The obtained reduced calcined sand is neutrally leached with sulfuric acid, the initial acidity of neutral leaching is 120g / L, the ratio of liquid to solid (volume / weight) is 5:1, the end point of neutral leaching is pH 5.4, and the neutral leaching time is 30 min, filtered to obtain neutral leaching liquid and middle leaching residue, neutral leaching leaching rate Zn75.9%, Fe53.3%.
[0039] 3. Carry out acid leaching of the middle leaching slag, the acid leaching initi...
Embodiment 3
[0045] A method for extracting indium zinc and preparing iron oxide from high-iron indium-zinc calcine, comprising the following steps:
[0046] 1. The high-iron zinc calcine containing Zn53.2%, Fe18.98%, In0.093% is reduced in contact with CO in the rotary kiln, and nitrogen gas containing 30% carbon monoxide is introduced, the excess coefficient is 2, and the reduction temperature is 700-730 ℃, the reduction time is 60min, and the Fe-containing 2+ 15.21% reduced calcine, the reduction rate is 80.1%.
[0047] 2. The obtained reduced calcined sand is neutrally leached with sulfuric acid, the initial acidity of neutral leaching is 100g / L, the ratio of liquid to solid (volume / weight) is 5:1, and the final pH is 5.4. Slag, neutral leaching Zn leaching rate of 69.38%, iron 55.8%.
[0048] 3. Carry out acid leaching of the middle leaching residue, the acid leaching initial acidity is 200g / L, the leaching temperature is 95°C, the acid leaching terminal acidity is 50g / L, and the le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com