Application of carbon-21 steride compound
A technology of steroidal compounds and drugs, applied in the field of medicine, can solve the problems of unreported application and little research on the pharmacological activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
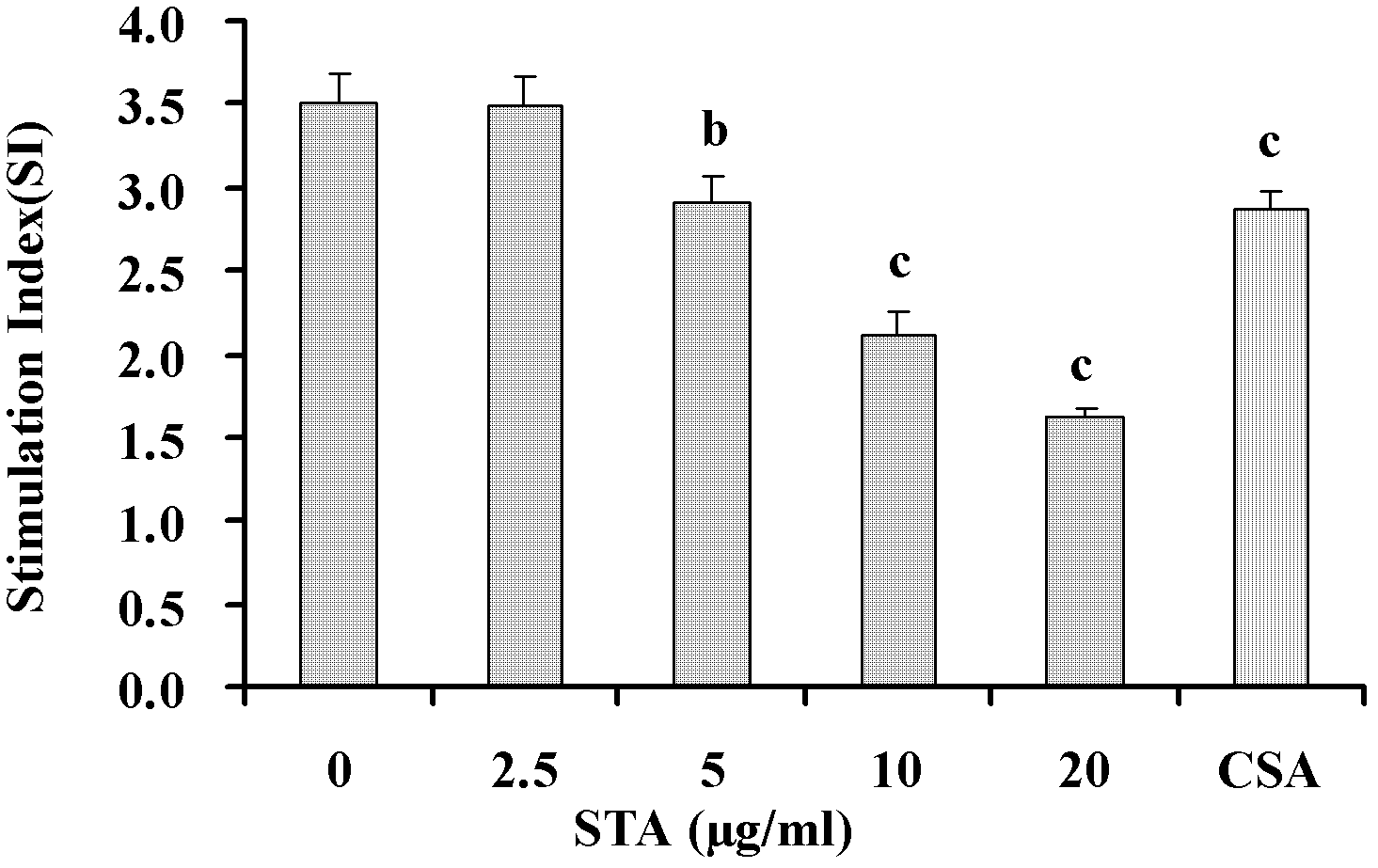
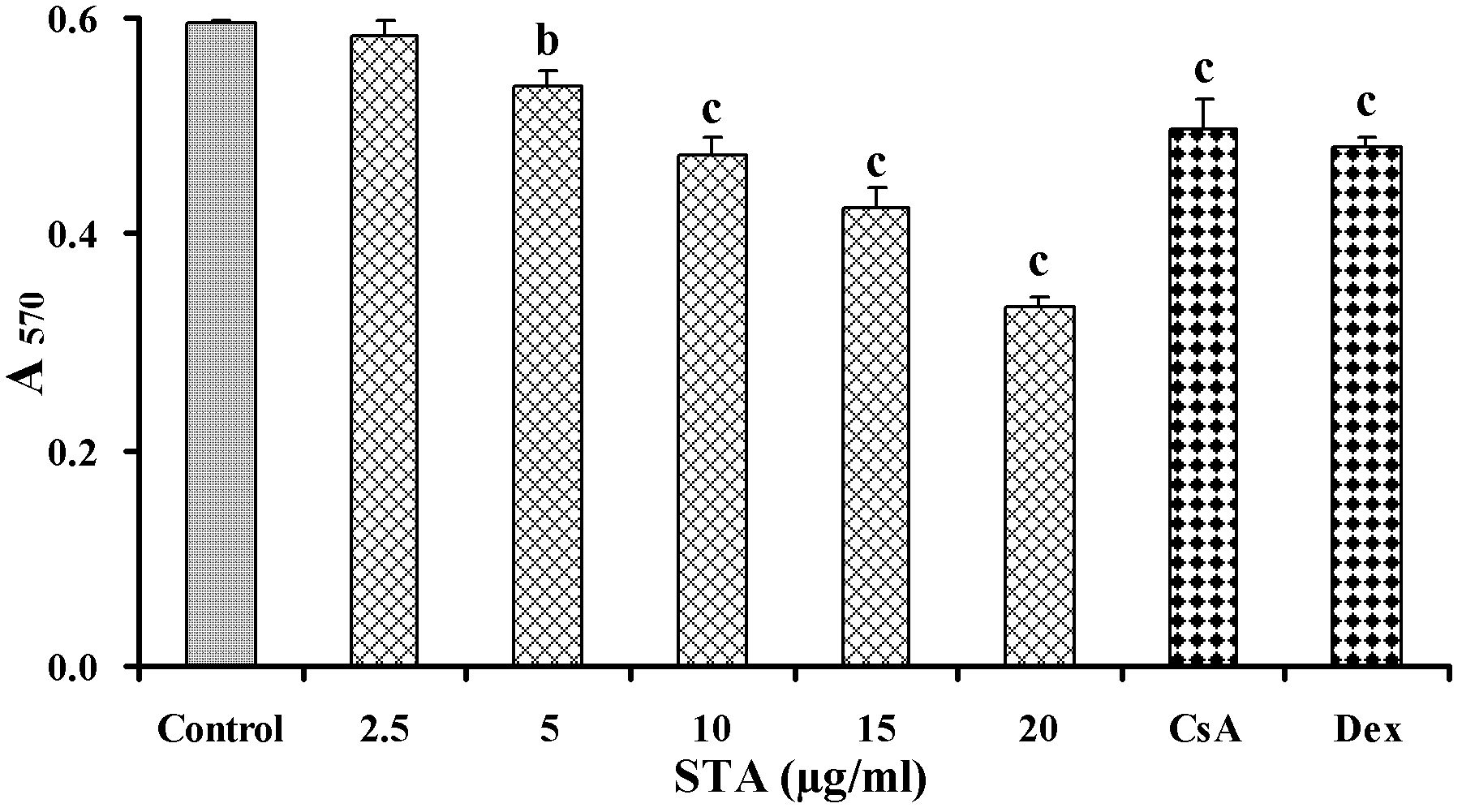
[0028] Example 1: Inhibition of STA on T lymphocyte activation in vitro
[0029] 1.1 Experimental method
[0030] 1.1.1 Preparation of mouse spleen cell suspension
[0031] The spleen of the mice was taken aseptically, added with appropriate amount of Hank's solution, and ground, filtered through a 200-mesh stainless steel mesh, centrifuged at 1500 rpm for 5 minutes, discarded the supernatant, red blood cells were removed with red blood cell lysate, and washed twice with Hank's solution. Collect spleen cells, add appropriate amount of RPMI 1640 complete culture medium to suspend, count with 0.4% (mass percentage) trypan blue exclusion method, and the number of viable cells is not less than 95%.
[0032] 1.1.2 T lymphocyte separation
[0033] T cells were separated by nylon wool column method. Take the sterilized nylon wool column and fix it vertically, put it in the ultra-clean table, connect the rubber tube and valve controller at the bottom outlet; flush the balanced column bed with...
Embodiment 2
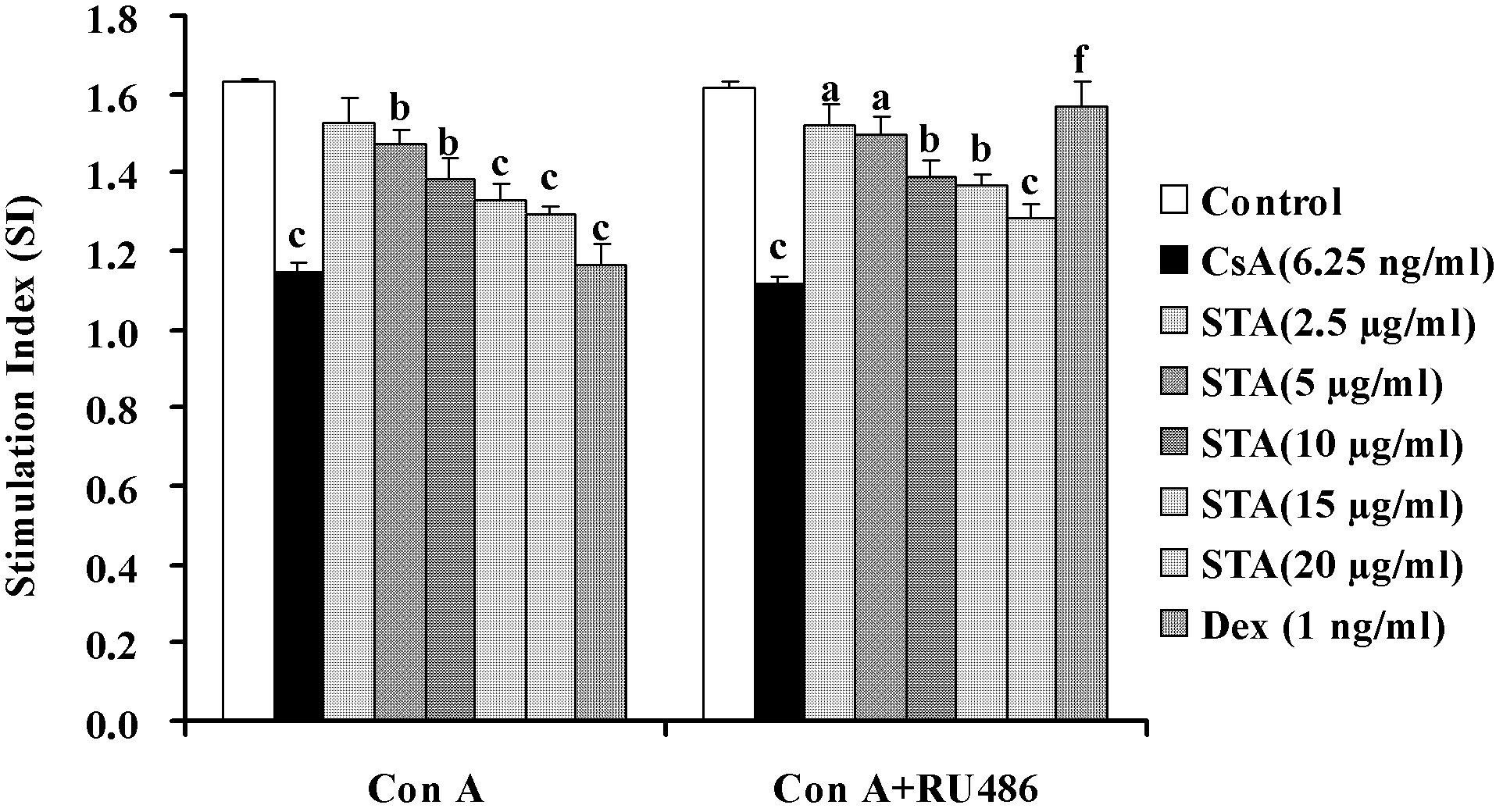
[0056] Example 2: Inhibition of T lymphocyte activation by STA in vivo
[0057] DTH response is an immune response mediated by sensitized T cells. It is a local reactive inflammation characterized by mononuclear cell infiltration and cell degeneration and necrosis caused by the specific reaction of T cells with antigens, through phagocytes and lymphocytes Gathering in local tissues, causing local damage and increased vascular permeability, such as increased ear swelling. Therefore, we used the DTH response induced by DNFB to observe the effect of STA on the activation of T lymphocytes in vivo.
[0058] 2.1 Experimental method
[0059] 2.1.1 Preparation of drugs
[0060] The drug STA and the positive control CsA are both polished with 0.5% (mass percentage) CMC-Na solution and diluted to the required concentration.
[0061] 2.1.2 Grouping and processing
[0062] Take 50 mice, shave their abdomen, apply 50μl of 1% (mass percentage) DNFB acetone sesame oil (1∶1, volume ratio) solution to ...
Embodiment 3
[0069] tablet:
[0070]
[0071] Preparation method: mix the active ingredient, lactose and starch, evenly moisten it with water, sieve and dry the moistened mixture, then sieve, add magnesium stearate, and then press the mixture into tablets, each weighs 250mg, and the active ingredient content is 10mg .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com