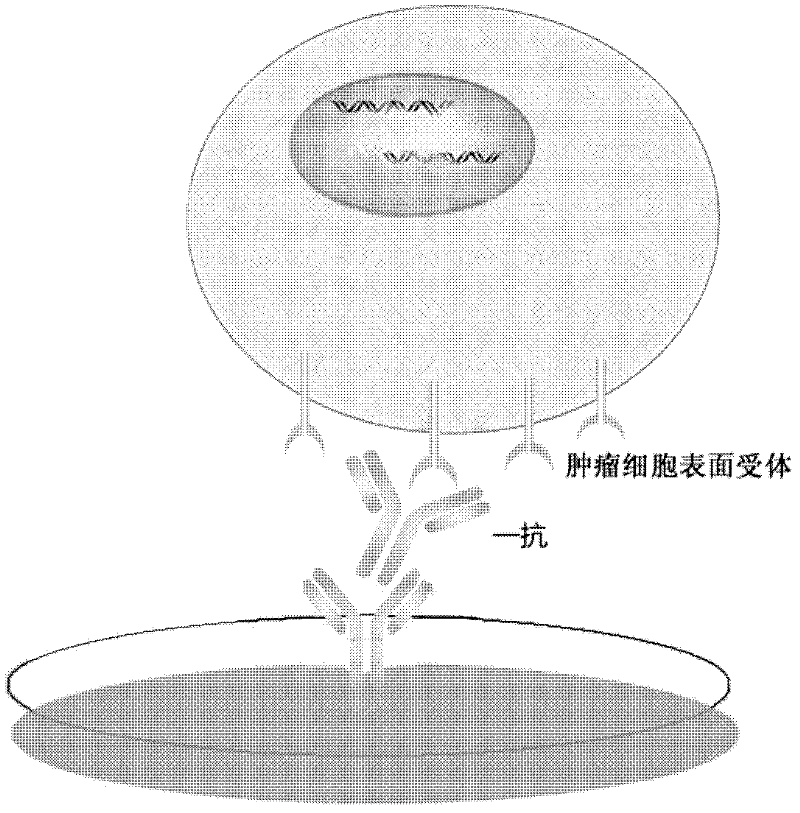
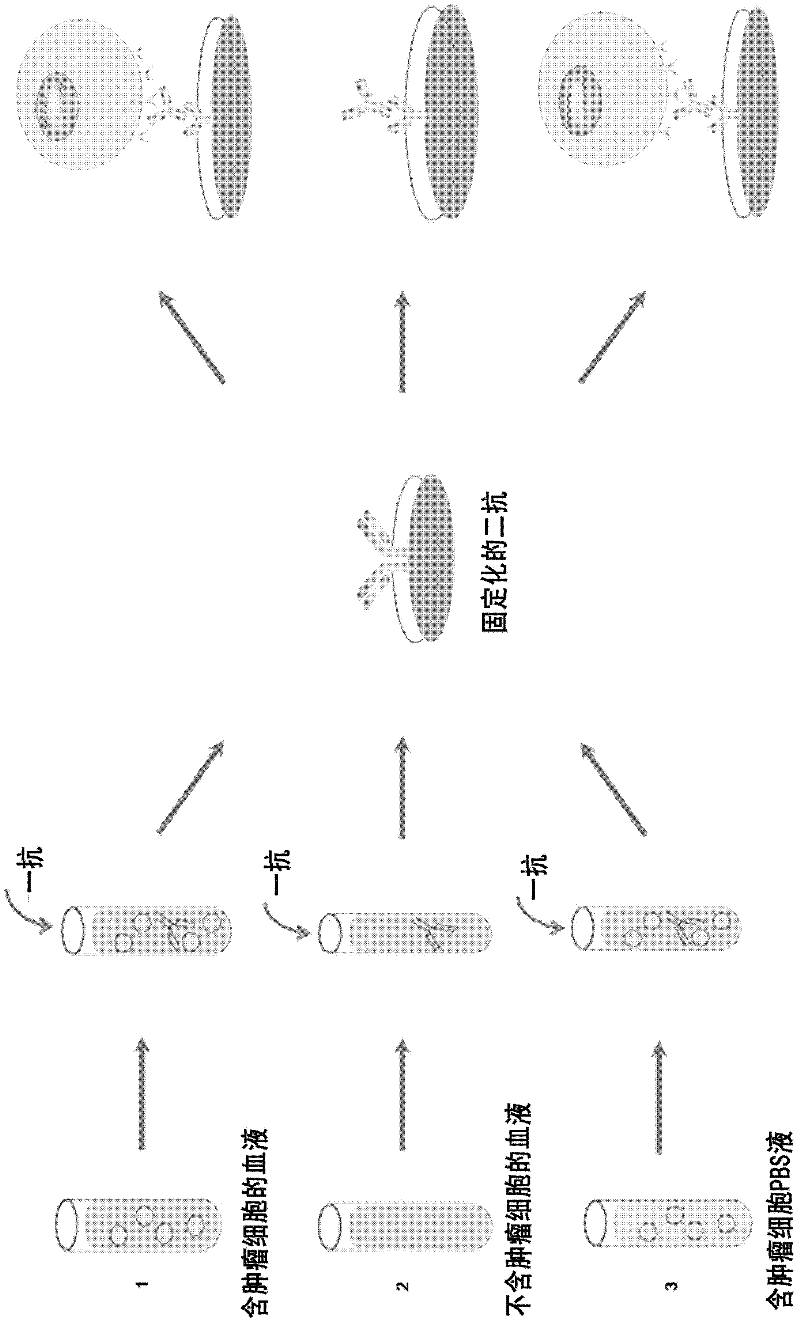
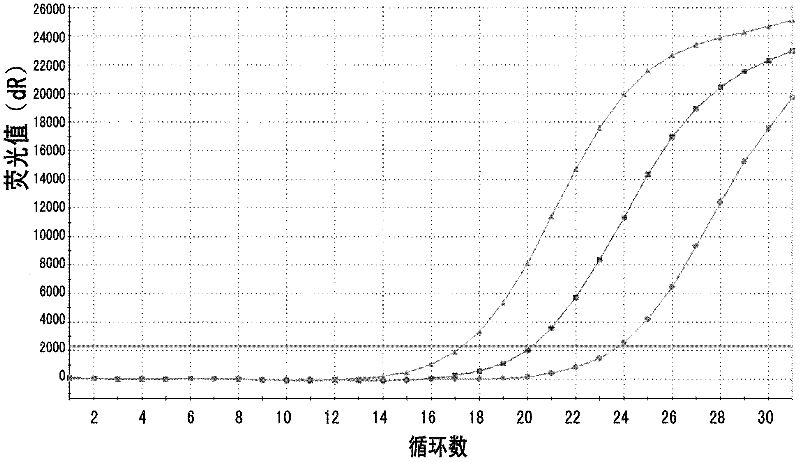
Method for enriching specificity of circulating tumor cell
A tumor cell enrichment technology, applied in the direction of tumor/cancer cells, animal cells, vertebrate cells, etc., can solve the problem of inability to meet clinical rapid detection and actual detection, inability to specifically identify tumor cells, insufficient sensitivity of detection, etc. problems, to achieve the effect of convenient and fast enrichment, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] (a) Preparation of rabbit anti-human cell surface receptor primary antibody
[0047] Dissolve 200 μg of antigen in 1 mL of PBS buffer solution, take 1 mL of the above antigen and add conventional 1 mL of complete Freund's adjuvant, shake vigorously to fully emulsify it, and prepare antigen emulsion. The above-mentioned emulsified antigen was subcutaneously injected into the rabbit's back, with an injection volume of about 500 μL per point. After the first immunization, the antigen was injected every 2-3 weeks for booster immunization, and the blood was collected 7-14 days after the last immunization. The collected blood was placed in a 37°C incubator for about half an hour, and then placed at 4°C overnight, centrifuged at 10,000g for 10 minutes at 4°C, and the supernatant was collected as antiserum, and the above antiserum was subjected to affinity column chromatography Purification was carried out to obtain the primary antibody, which was then stored in a refrigerator...
Embodiment 1
[0080] In this example, tumor cell Epidermal Growth Factor Receptor (EGFR) was used as the specific binding target, and EGFR was used as the antigen to prepare the first antibody and the second antibody, and the tumor cell H1975 and healthy human blood were used as the medium to capture Taking tumor cells as an example, the enrichment and detection system of the present invention is further described and verified. The method of enrichment and detection comprises the following steps:
[0081] 1. Preparation of EGFR primary antibody and secondary antibody
[0082] (a) Preparation of rabbit anti-human EGFR primary antibody
[0083] Dissolve 200 μg of antigen in 1 mL of PBS buffer solution, take 1 mL of the above antigen and add conventional 1 mL of complete Freund's adjuvant, shake vigorously to fully emulsify it, and prepare antigen emulsion. The above-mentioned emulsified antigen was subcutaneously injected into the rabbit's back, and the injection volume was about 500 μL. A...
Embodiment 2
[0116] This example is basically the same as Example 1, except that in this example, there is only the primary antibody but no secondary antibody.
[0117] 1. Preparation of EGFR primary antibody
[0118] (a) Preparation of rabbit anti-human EGFR primary antibody
[0119] Dissolve 200 μg of antigen in 1 mL of PBS buffer solution, take 1 mL of the above antigen and add conventional 1 mL of complete Freund's adjuvant, shake vigorously to fully emulsify it, and prepare antigen emulsion. The above-mentioned emulsified antigen was subcutaneously injected into the rabbit's back, and the injection volume was about 500 μL. After the first immunization, the antigen was injected every 2-3 weeks for booster immunization, and the blood was collected 7-10 days after the injection. The collected blood was placed in a 37°C incubator for half an hour, and placed at 4°C overnight; then centrifuged at 10,000g at 4°C for 10 minutes, and the supernatant was collected as antiserum, which was pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com