Method for determining coagulation inhibitors
A technology of inhibitors and coagulation factors, which is applied in the field of coagulation diagnostics and can solve problems such as technical complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: Synthesis of Biotinyl-Ttds-Peptide Aldehyde Ligands for Thrombin and Factor Xa
[0150] The peptide aldehydes -D-Phe-Pro-Arg-H (see Claeson, G., Blood Coagulation and Fibrinolysis 5, 1994, p. 417) and -D-Arg-Gly-Arg-H (see Claeson , G., Blood Coagulation and Fibrinolysis 5, 1994, p. 426), which extends the Ttds spacer (Ttds = 4,7,10-trioxa-1,13-tridecanediamino-succinic acid (Bartos , A. et al., 2009, Biopolymers 92(2), 110-115)), and has biotin. The compound biotinyl-Ttds-D-Phe-Pro-Arg-H has a molecular weight of 930.15 g / mole and is hereinafter abbreviated as B-f-P-R-H. The compound biotinyl-Ttds-D-Arg-Gly-Arg-H has a molecular weight of 899.10 g / mole and is hereinafter abbreviated as B-r-G-R-H. Peptide aldehydes were removed from the solid phase with trifluoroacetic acid. Compounds were stored in lyophilized form at -20°C. The structure of the biotin linker is in figure 2 described in.
Embodiment 2
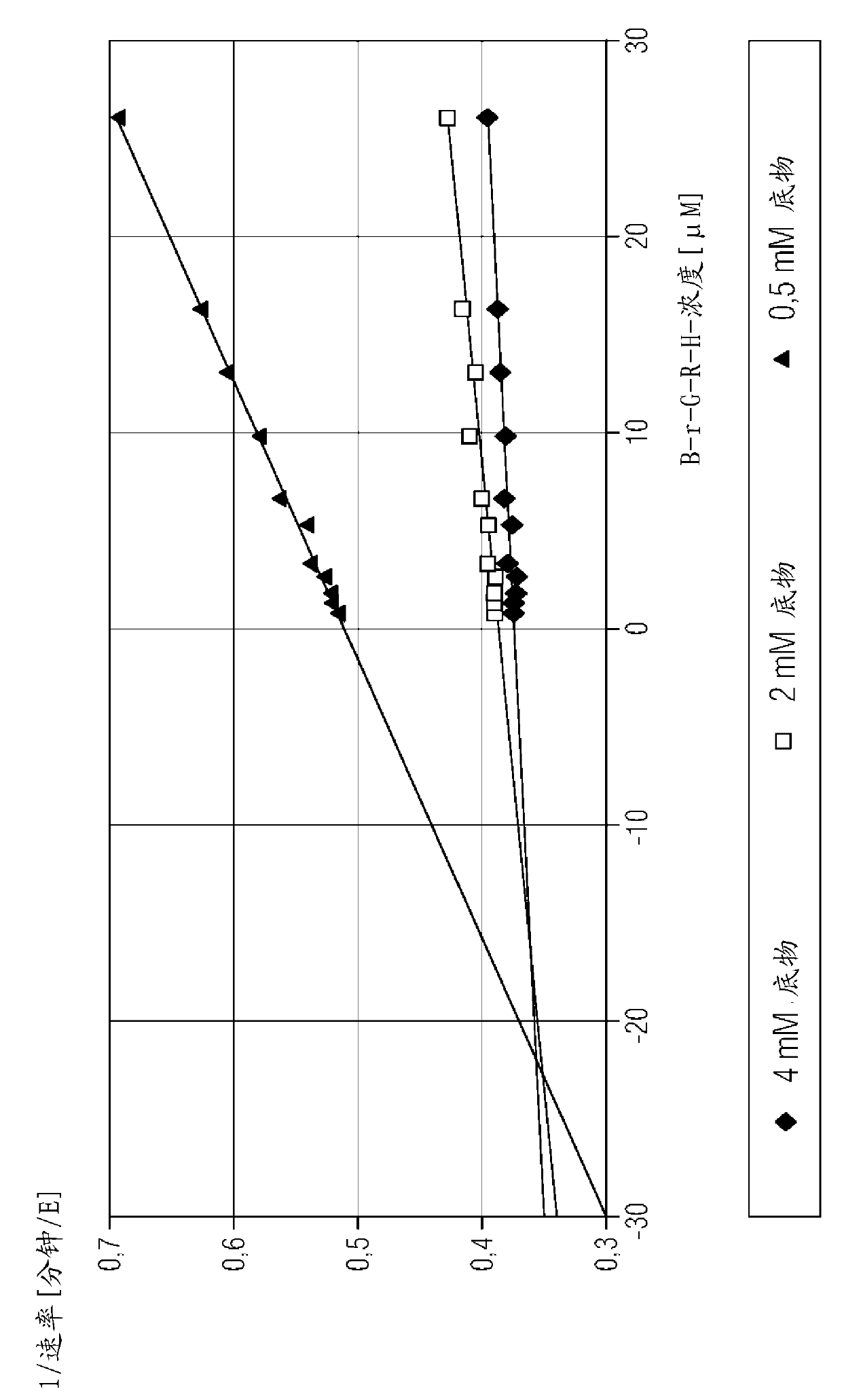
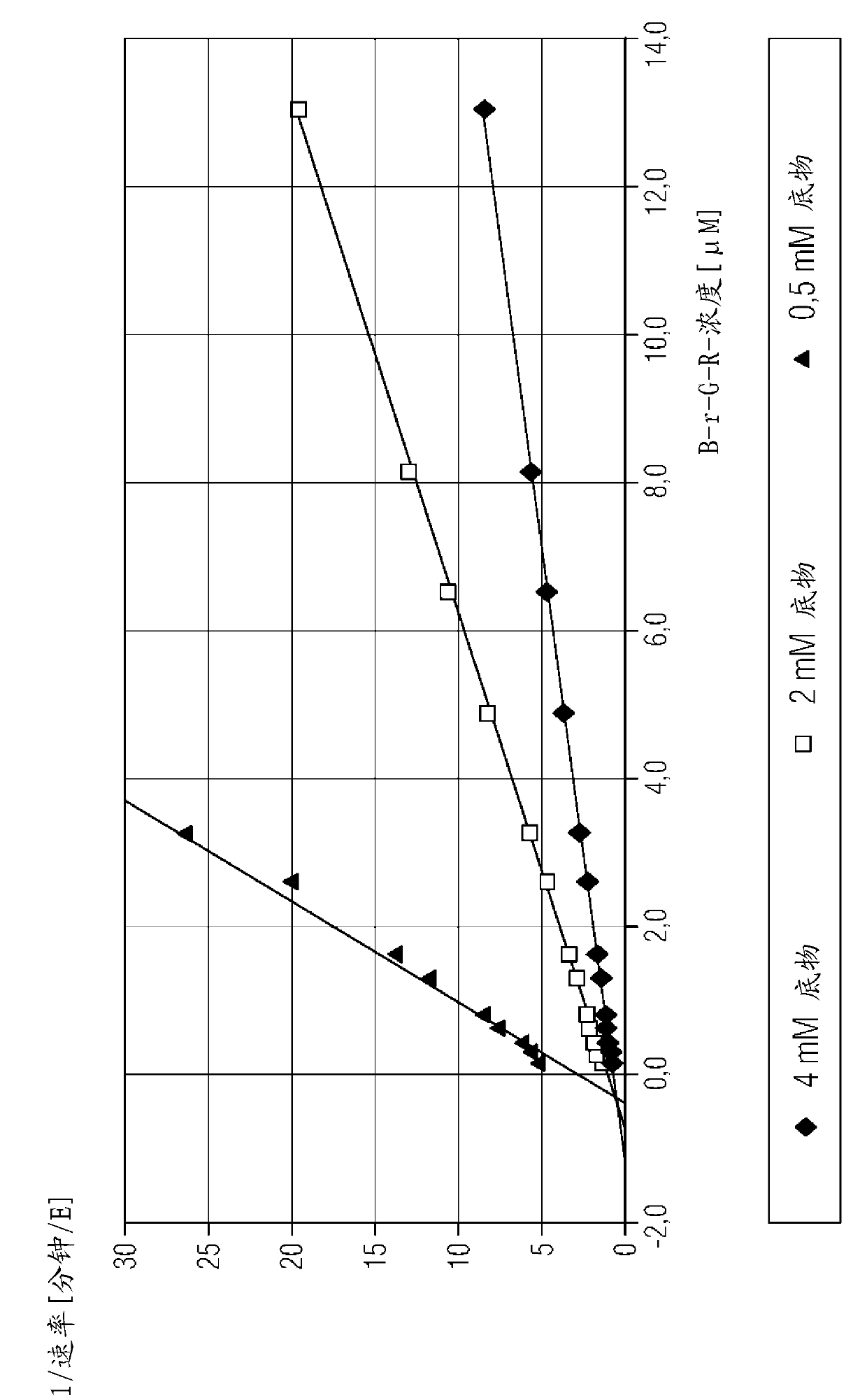
[0151] Example 2: Determination of the thrombin binding constant of the peptide aldehyde ligand
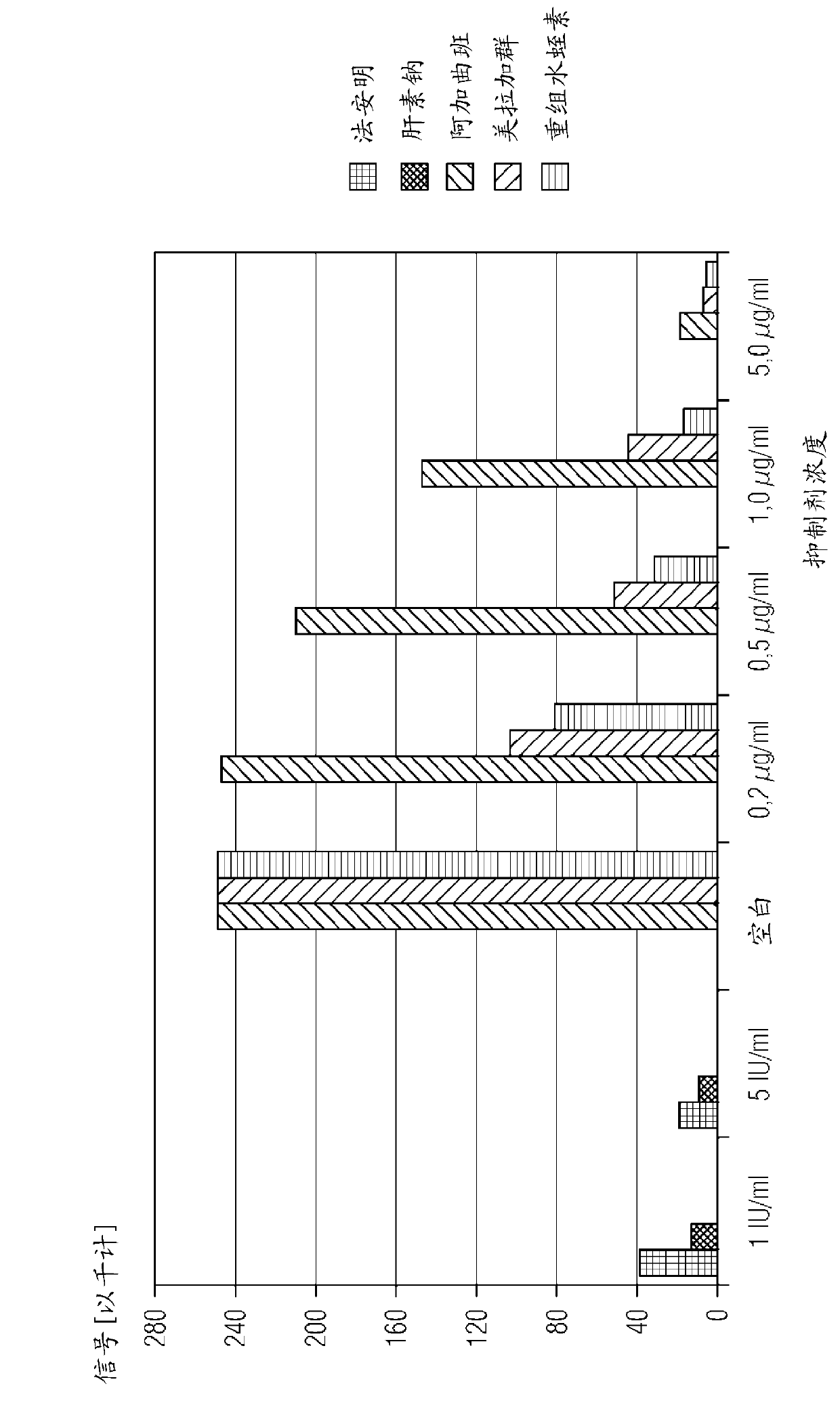
[0152] Kinetic data for peptide aldehyde ligands are determined in a chromogenic assay format by measuring the chromogenic peptide substrate that competes with the peptide aldehyde ligand for binding to the active site of a specific enzyme at varying substrate concentrations and peptide aldehyde-ligand concentrations. The hydrolysis rate of the substance is carried out. Binding constants (Ki) were determined by known methods (Dixon, M., 1953, Biochem J, 55, 170-171).
[0153] Thrombin binding constants were determined using the hirudin activity assay reagent from Siemens Healthcare Diagnostics. The hirudin activity assay contains lyophilized thrombin reagent (which consists of bovine thrombin, heparin inhibitor, and aprotinin), and lyophilized chromogenic substrate reagent (which has 4 mmol / l tos- Gly-Pro-Arg-ANBA-IPA (concentration of tosylglycyl-L-propyl-arginyl-5-amino-2-nitr...
Embodiment 3
[0172] Example 3: Determination of the F Xa Binding Constant of Peptide Aldehyde Ligands
[0173] Berichrom using Siemens Healthcare Diagnostics ? Reagents for the heparin assay determine the F Xa binding constant. Berichrom ? The heparin assay consists of the following reagents:
[0174] ● F Xa reagent, which consists of lyophilized plasma fractions containing Factor Xa and additives such as Tris, NaCl, EDTA and preservatives,
[0175] ● Chromogenic substrate reagent (Z-D-Leu-Gly-Arg-ANBA-methyl-amide),
[0176] ● dilution reagents for reconstitution, and
[0177] ● Dextran sulfate reagent, which consists of lyophilized dextran sulfate.
[0178]After reconstitution in 10 ml of diluent reagent, the concentration of dextran sulfate is 0.02 g / l. Reconstitute FXa reagent with reconstituted dextran sulfate reagent. The substrate reagent contained 4 mmol / l Z-D-Leu-Gly-Arg-ANBA-methyl-amide after making up with 2 ml deionized water. Prepare dilutions of substrate reagents wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com