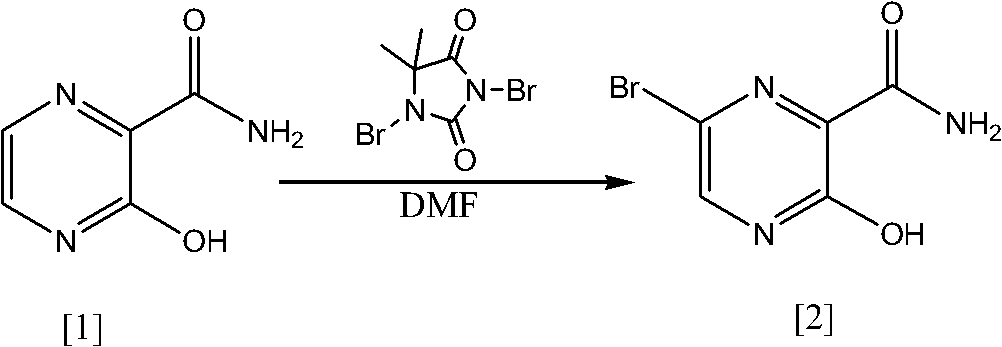
Preparation method of 6-bromine-3-hydroxyl-2-pyrazinamide
A technology of pyrazinamide and hydroxyl, which is applied in the field of preparation of 6-bromo-3-hydroxy-2-pyrazinamide, can solve the problems of low total yield, difficult removal of solvent, and high purchase cost, and achieve convenient market purchase, Wide range of solvents and easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Add 458g (3mol, 1eq) of 3-hydroxyl-2-pyrazinecarboxylate methyl ester and 532g (3mol, 1eq) of N-bromosuccinimide successively into 5L of acetonitrile, at 25°C Stir for 12 hours, after TLC detects that the reaction is complete, stop the reaction, filter, and the filter cake is vacuum-dried at normal temperature to obtain 554g of product 6-bromo-3-hydroxyl-2-pyrazinecarboxylic acid methyl ester (including its tautomer 6 -Bromo-3-oxo-3,4-dihydro-2-pyrazinecarboxylic acid methyl ester), the yield was 85%. The nuclear magnetic resonance of 6-bromo-3-hydroxyl-2-pyrazinecarboxylate methyl ester and mass spectrometry detection index are as follows:
[0021] 1 H-NMR (CDCl 3 , 600MHz) δ value: 4.09 (3H, s, CH 3 ), 8.53 (1H, s, pyrazine H)
[0022] MS (ESI) m / z: 233.1 [M+H] + , 235.2[M+2+H] +
[0023] (2) Dissolve 21g (0.14mol) of 6-bromo-3-hydroxyl-2-pyrazinecarboxylic acid methyl ester in 450mL of tetrahydrofuran, add 257ml of concentrated ammonia (mass fraction 25%) ...
Embodiment 2
[0027] (1) Add 308g (2mol, 1eq) of methyl 3-hydroxy-2-pyrazinecarboxylate and 956g (6mol, 3eq) of liquid bromine into 4L of acetonitrile, stir for one hour at 27°C, and TLC detects that the reaction is complete , the reaction solution was poured into 4L of water, and the excess liquid bromine was removed with a saturated aqueous solution of sodium sulfite, left to filter, and the filter cake was vacuum-dried at room temperature to obtain 300 g of the product 6-bromo-3-hydroxyl-2-pyrazinecarboxylic acid methyl Esters (including its tautomer methyl 6-bromo-3-oxo-3,4-dihydro-2-pyrazinecarboxylate) in 65% yield. The nuclear magnetic resonance of 6-bromo-3-hydroxyl-2-pyrazinecarboxylate methyl ester and mass spectrometry detection index are as follows:
[0028] 1 H-NMR (CDCl 3 , 600MHz) δ value: 4.09 (3H, s, CH 3 ), 8.53 (1H, s, pyrazine H), 11.45 (1H, s, OH)
[0029] MS (ESI) m / z: 233.1 [M+H] + , 235.2[M+2H] (2+) , 255.1[M+Na] +
[0030] (2) Dissolve 10g (0.04mol) of 6-bro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com