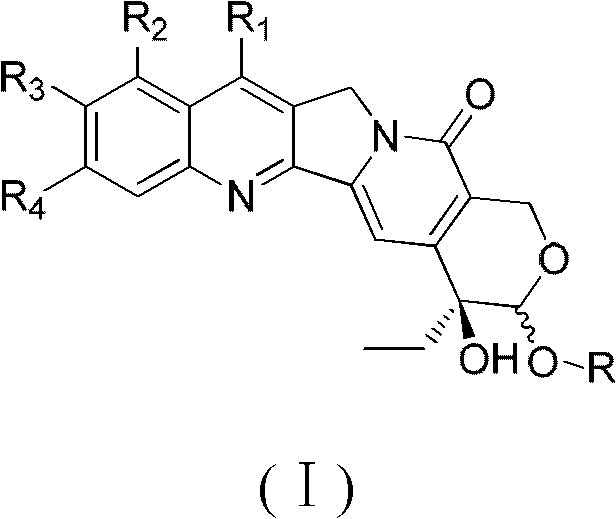
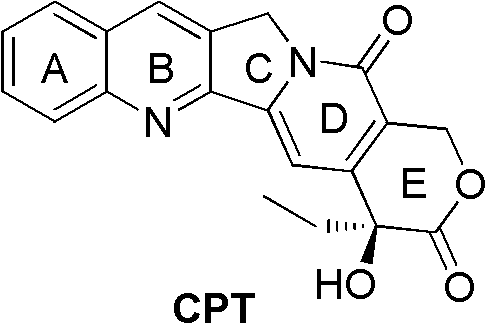
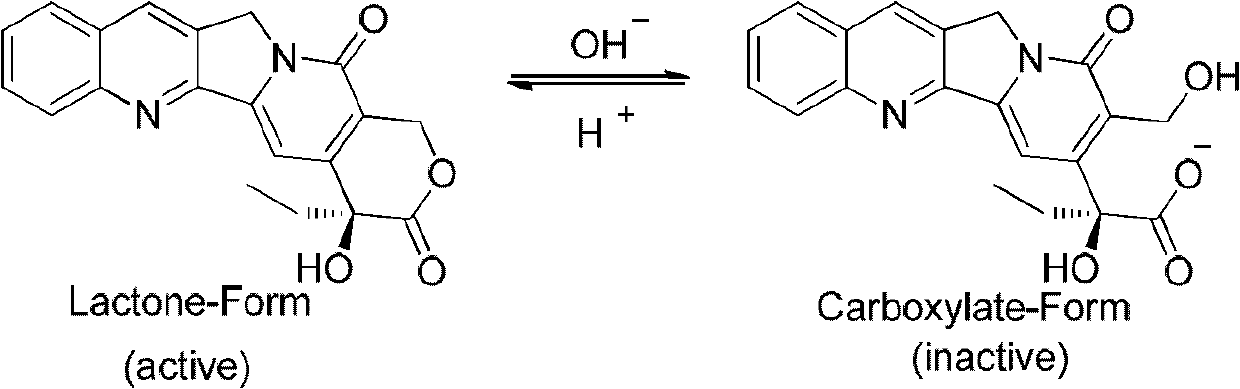
Camptothecin E ring analogues and applications thereof as drugs
A technology of camptothecin and analogs, applied in the field of camptothecin E-ring analogs and their use as medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、21
[0074] Embodiment 1, 21-hydroxycamptothecin
[0075] Suspend 1.00g of camptothecin in 50mL of methanol, stir at room temperature for 5 minutes, add 0.30g of potassium borohydride, and continue to react for 1h at room temperature. Make it dissolve, and then adjust the pH to be acidic with acetic acid in an ice-water bath. At this time, a large amount of solid precipitates, which is filtered, washed with water, and vacuum-dried to obtain 0.99 g of light yellow solid 21-hydroxycamptothecin (98.4%).
[0076] 1 H-NMR (DMSO):
[0077] 0.91(t, 3H), 1.72(q, 2H), 4.49-4.63(q, 2H), 4.94(s, 1H), 5.00(d, 1H), 5.25(s, 2H), 6.73(d, 1H) , 7.38(s, 1H), 7.69(t, 1H), 7.85(t, 1H), 8.11(d, 1H), 8.16(d, 1H), 8.66(s, 1H).
Embodiment 2、10
[0078] The synthesis of embodiment 2,10-methoxy-21-hydroxycamptothecin
[0079] Suspend 1.00 g of 10-methoxycamptothecin in 50 mL of methanol, stir at room temperature for 5 minutes, add 0.30 g of potassium borohydride, and continue to react for 1 h at room temperature. At this time, the reaction solution is clear, and the solvent is evaporated to obtain light green Solid, add 50mL of water to dissolve it, then adjust the pH to acidic with acetic acid in an ice-water bath, at this time a large amount of solid precipitates, filter and wash with water, and dry in vacuo to obtain 0.96g of light yellow solid 10-methoxy-21-hydroxycamptotheca Alkali (95.4%).
[0080] 1 H-NMR (DMSO):
[0081] 0.90(t, 3H), 1.70(q, 2H), 3.93(s, 3H), 4.46-4.64(q, 2H), 4.91(s, 1H), 4.97(d, 1H), 5.21(s, 2H) , 6.74(d, 1H), 7.30(s, 1H), 7.47(d, 1H), 7.50(s, 1H), 8.05(d, 1H), 8.51(s, 1H).
Embodiment 3
[0082] The synthesis of embodiment 3,9-nitro-21-hydroxycamptothecin
[0083] Suspend 1.00 g of 9-nitrocamptothecin in 50 mL of methanol, stir at room temperature for 5 minutes, add 0.30 g of potassium borohydride, and continue to react for 1 h at room temperature. At this time, the reaction solution is clear, and the solvent is evaporated to obtain a light green solid , add 50mL of water to make it dissolve, then adjust PH to be acidic with acetic acid under ice-water bath, at this moment, separate out a large amount of solids, wash with suction filtration, dry in vacuo to obtain 0.95g light yellow solid 21-hydroxycamptothecin (94.5%).
[0084] 1 HNMR (DMSO):
[0085] 0.91(t, 3H), 1.72(q, 2H), 4.70-4.81(q, 2H), 4.94(s, 1H), 5.14(d, 1H), 5.27(s, 2H), 6.77(d, 1H) , 7.44(s, 1H), 8.03(t, 1H), 8.52(t, 1H), 8.57(d, 1H), 9.15(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com