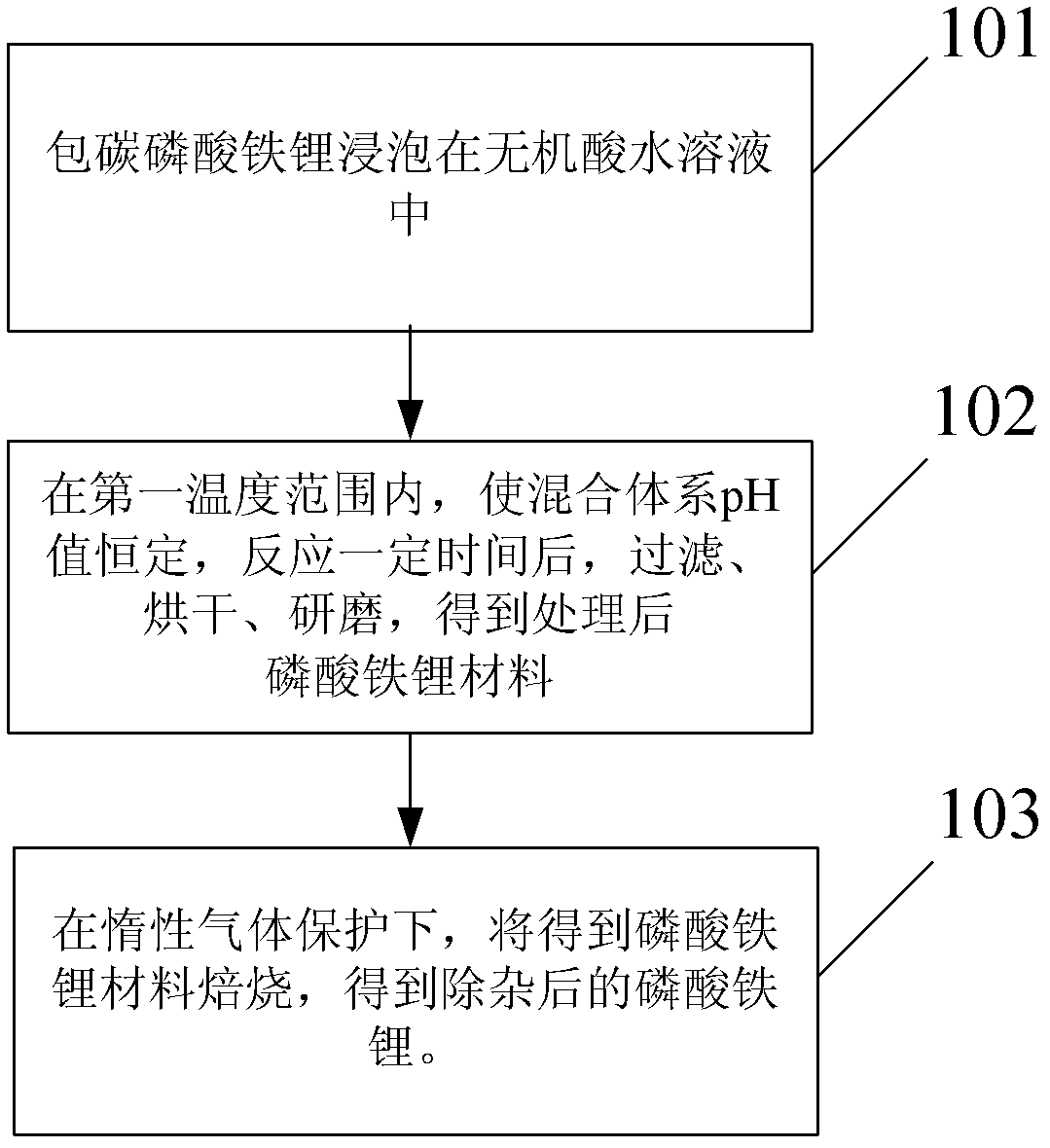
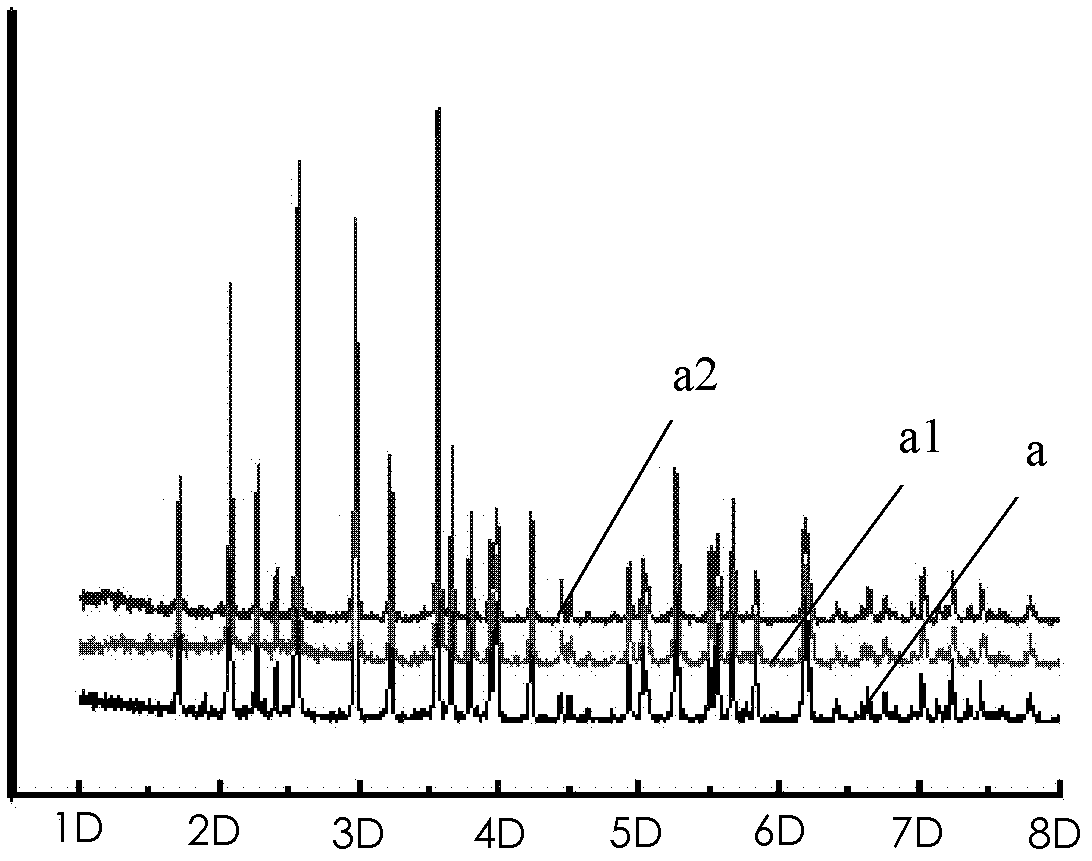
Method for removing impurities from lithium iron phosphate (LiFePO4) and LiFePO4 battery
A technology of lithium iron phosphate and carbon-coated lithium iron phosphate, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of product residue, damage to material structure, and failure to fundamentally suppress it, so as to reduce self-discharge ability and eliminate Black spots, the effect of improving battery safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 10.00g of carbon-coated lithium iron phosphate from the domestic manufacturer a, soak it in 120ml of HF aqueous solution with a pH of 3.5, stir rapidly, and keep it warm at 40°C to detect the change of the pH value of the system. When the pH rises to 4, drop Add HF aqueous solution with a pH value of 2 to keep the pH of the reaction system at 4.0, react for 100 h, and monitor the iron content in the solution. Then filter, wash the filtered residue with absolute ethanol, wash three times, dry at 100°C for 6h, dry, grind to obtain the treated material, and then place the treated material in a muffle furnace under argon protection Calcined at 100°C for 6 hours to obtain lithium iron phosphate material.
Embodiment 2
[0043] Weigh 10.00g of carbon-coated lithium iron phosphate from domestic manufacturer a, soak it in 120ml of HCl aqueous solution with a pH of 4.0, stir rapidly, and keep it warm at 60°C to detect the change of the pH value of the system. When the pH rises to 4.5, add dropwise HCl aqueous solution with a pH value of 3 keeps the pH of the reaction system constant at 4.5, reacts for 40 hours, and monitors the iron content in the solution after soaking. Then filter, wash the filtered residue with anhydrous methanol, wash four times, dry at 100°C for 6h, dry, grind to obtain the treated material, and then place the treated material in a muffle furnace under nitrogen protection Calcined at 600°C for 1 hour to obtain lithium iron phosphate material.
Embodiment 3
[0045] Weigh 10.00 g of carbon-coated lithium iron phosphate from domestic manufacturer b and soak it in 120 ml of H with a pH of 3.5. 3 PO 4 In the aqueous solution, stir rapidly and keep warm at 80°C to detect the change of the pH value of the system. When the pH value rises to 4, add dropwise H 3PO 4 aqueous solution, keep the pH of the reaction system constant at 4, react for 6 hours, and monitor the iron content in the solution after soaking. Then filter, wash the filtered residue with diethyl carbonate (DEC), wash three times, dry at 100°C for 6h, dry, grind to obtain the treated material, and then place the treated material in a muffle furnace , and roasted at 200°C for 3h under the protection of nitrogen to obtain lithium iron phosphate material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com