Food additive and preparation method thereof
A food additive, cinnamic acid technology, applied in food preservation, food science, edible seed preservation and other directions, to achieve the effect of improving fat solubility and obvious antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
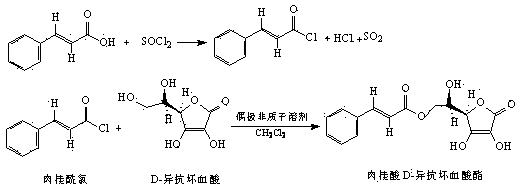
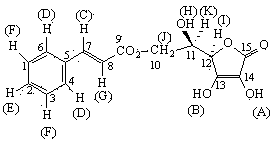
[0031] Place the reaction flask containing 29.63 g (200 mmol) of cinnamic acid in an ice-water bath, and slowly add 28.4 mL (560 mmol) of SOCl 2 , after that, stir and react at 95-100°C for 2h, after the reaction is completed, distill SOCl at atmospheric pressure 2 , and then distilled under reduced pressure to remove SOCl as much as possible 2 , the cinnamoyl chloride obtained is directly used in the next step reaction;
[0032] Add 42.28 g (240 mmol) D-isoascorbic acid and 127 mL DMAc (dried) into a three-neck flask, turn on magnetic stirring, and after D-isoascorbic acid is completely dissolved, adjust the reaction temperature to 50 ° C, within 2 hours from constant pressure to The cinnamoyl chloride prepared above and 40 mL of CH were slowly added dropwise into the dropping funnel 2 Cl 2 (dried) mixed solution; after the dropwise addition, continue to react for 8 h;
[0033] After the reaction is completed, cool the reaction mixture to room temperature, add 180 mL ethy...
Embodiment 2
[0035] The preparation of cinnamoyl chloride is with embodiment 1;
[0036] Add 42.28 g (240 mmol) D-erythorbic acid and 115 mL DMF (dried) into a three-neck flask, turn on the magnetic stirring, and after the D-erythorbic acid is completely dissolved, adjust the reaction temperature to 20°C, within 3 hours from constant pressure to Slowly add the above-prepared cinnamoyl chloride and 90 mL CH 2 Cl 2 (dried) mixed solution; after dropwise addition, continue to react for 12 h;
[0037] After the reaction is completed, cool the reaction mixture to room temperature, add 180 mL of ethyl acetate and 180 ml of distilled water, stir and extract, separate the organic phase and the water phase; extract the water phase twice with an appropriate amount of ethyl acetate, combine the organic phases, and wash with distilled water From the combined organic phases to the separated aqueous layer, there was no D-isoascorbic acid residue, the organic phase was dried over anhydrous sodium sulfa...
Embodiment 3
[0039] The preparation of cinnamoyl chloride is with embodiment 1;
[0040] Add 42.28 g (240 mmol) D-erythorbic acid and 115 mL DMAc (dried) into a three-neck flask, turn on the magnetic stirring, and after the D-erythorbic acid is completely dissolved, adjust the reaction temperature to 35°C, within 3 hours from constant pressure to The cinnamoyl chloride prepared above and 58 mL of CH were slowly added dropwise into the dropping funnel 2 Cl 2 (dried) mixed solution; after the dropwise addition, continue to react for 8 h;
[0041] After the reaction was completed, the reaction mixture was cooled to room temperature, 200 mL ether and 200 ml distilled water were added, stirred and extracted, the organic phase and the water phase were separated, the water phase was extracted twice with an appropriate amount of ether, and the organic phases were combined. The combined organic phases were washed with distilled water until no D-isoascorbic acid remained in the separated aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com