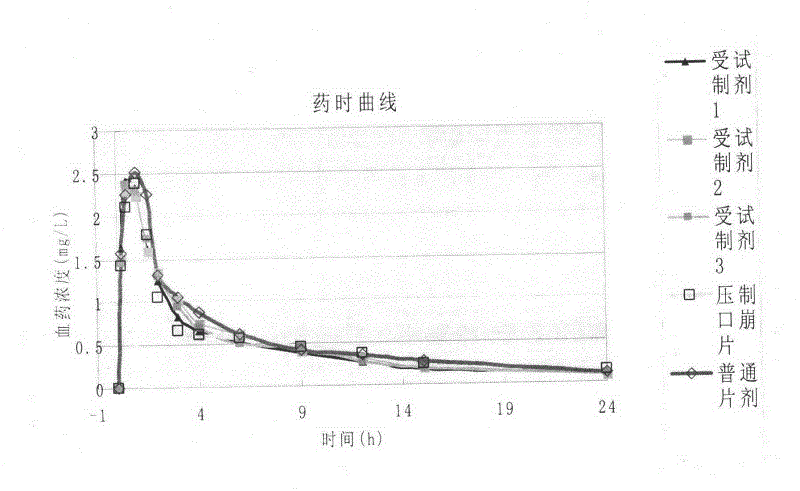
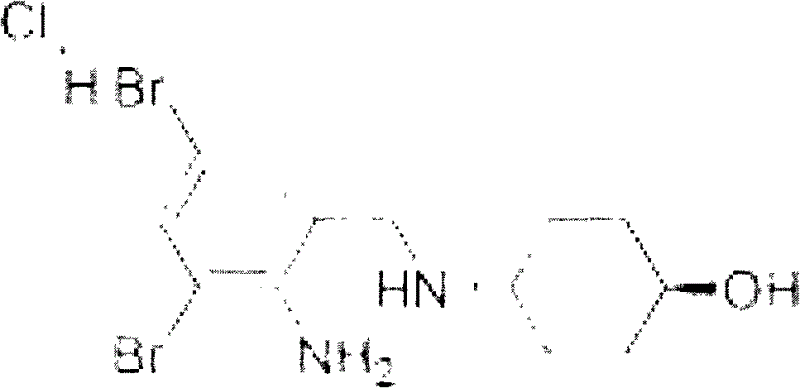
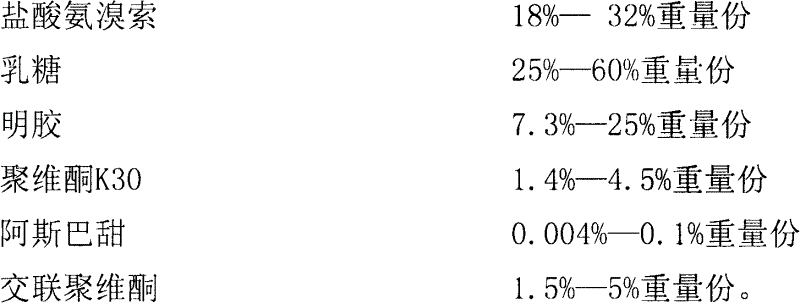Ambroxol hydrochloride composition
A technology of ambroxol hydrochloride and composition, applied in the field of ambroxol hydrochloride composition freeze-dried orally disintegrating tablet and its preparation, can solve the problem of affecting the therapeutic effect of ambroxol hydrochloride, long disintegration time of ambroxol hydrochloride, Children and the elderly have difficulty swallowing and other problems, and achieve the effects of improving bioavailability, rapid drug onset, and shortening dissolution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of ambroxol hydrochloride composition freeze-dried orally disintegrating tablets,
[0052] Prescription: 1000 tablets
[0053]
[0054] Preparation Process:
[0055] Dissolve the prescribed amount of povidone K30, crospovidone, lactose, and aspartame in purified water of 10%-15% of the total prescription water, and add the prescribed amount of gelatin to 10% of the total prescription water. 10%-15% purified water, heated to boiling and dissolved under stirring, dissolved the prescribed amount of ambroxol hydrochloride in purified water with prescribed water consumption of 25%-30%, combined the raw materials and auxiliary materials, and continued to stir for 3 Hour, measure PH, adjust PH to 6.0 with the hydrochloric acid solution of 2mol / L, according to the ambroxol hydrochloride composition freeze-drying orally disintegrating tablet specification, after determining the loading amount, the medicinal liquid after merging is subpackaged according to the loa...
Embodiment 2
[0057] Preparation of ambroxol hydrochloride composition freeze-dried orally disintegrating tablets,
[0058] Prescription: 1000 tablets
[0059] Prescription: 1000 tablets
[0060]
[0061] Preparation Process:
[0062] Dissolve the prescribed amount of povidone K30, crospovidone, lactose, and aspartame in purified water of 10%-15% of the total prescription water, and add the prescribed amount of gelatin to 10% of the total prescription water. 10%-15% purified water, heated to boiling and dissolved under stirring, dissolved the prescribed amount of ambroxol hydrochloride in purified water with prescribed water consumption of 25%-30%, combined the raw materials and auxiliary materials, and continued to stir for 3 Hour, measure PH, adjust PH to 6.0 with the hydrochloric acid solution of 2mol / L, according to the ambroxol hydrochloride composition freeze-drying orally disintegrating tablet specification, after determining the loading amount, the medicinal liquid after mergin...
Embodiment 3
[0064] Preparation of ambroxol hydrochloride composition freeze-dried orally disintegrating tablets,
[0065] Prescription: 1000 tablets
[0066]
[0067]
[0068] Preparation Process:
[0069] Dissolve the prescribed amount of povidone K30, crospovidone, lactose, and aspartame in purified water of 10%-15% of the total prescription water, and add the prescribed amount of gelatin to 10% of the total prescription water. 10%-15% purified water, heated to boiling and dissolved under stirring, dissolved the prescribed amount of ambroxol hydrochloride in purified water with prescribed water consumption of 25%-30%, combined the raw materials and auxiliary materials, and continued to stir for 3 Hour, measure PH, adjust PH to 6.0 with the hydrochloric acid solution of 2mol / L, according to the ambroxol hydrochloride composition freeze-drying orally disintegrating tablet specification, after determining the loading amount, the medicinal liquid after merging is subpackaged accordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com