Artemisinin detrivative and application of its medicinal salt
A technology of artemisinin derivatives and medicinal salts, which can be used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve the problems of diseases affecting the survival rate of patients and poor prognosis of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 Preparation of artemisinin derivative biarteether amine maleate
[0017] In this embodiment, starting from the known compound hydroxyarteether (references: Li Ying et al., Acta Pharmaceutica Sinica, 1981, 16: 429-439), its p-toluenesulfonate was first prepared, and then in the solvent dimethylformamide React with ammonia water to obtain biarteether amine, the reaction route is:
[0018]
[0019] The obtained biarteetheramine is then re-formed into the maleate salt.
[0020] The specific operation is as follows:
[0021] Dissolve p-toluenesulfonate of hydroxyarteether (1.54g) in dimethylformamide (10mL), add ammonia (0.5mL) and stir to heat to 40-50°C, and react for about 20h. After TLC detected that the raw material spots basically disappeared, the reaction solution was poured into ice water, extracted repeatedly with ethyl acetate, the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was ...
Embodiment 2
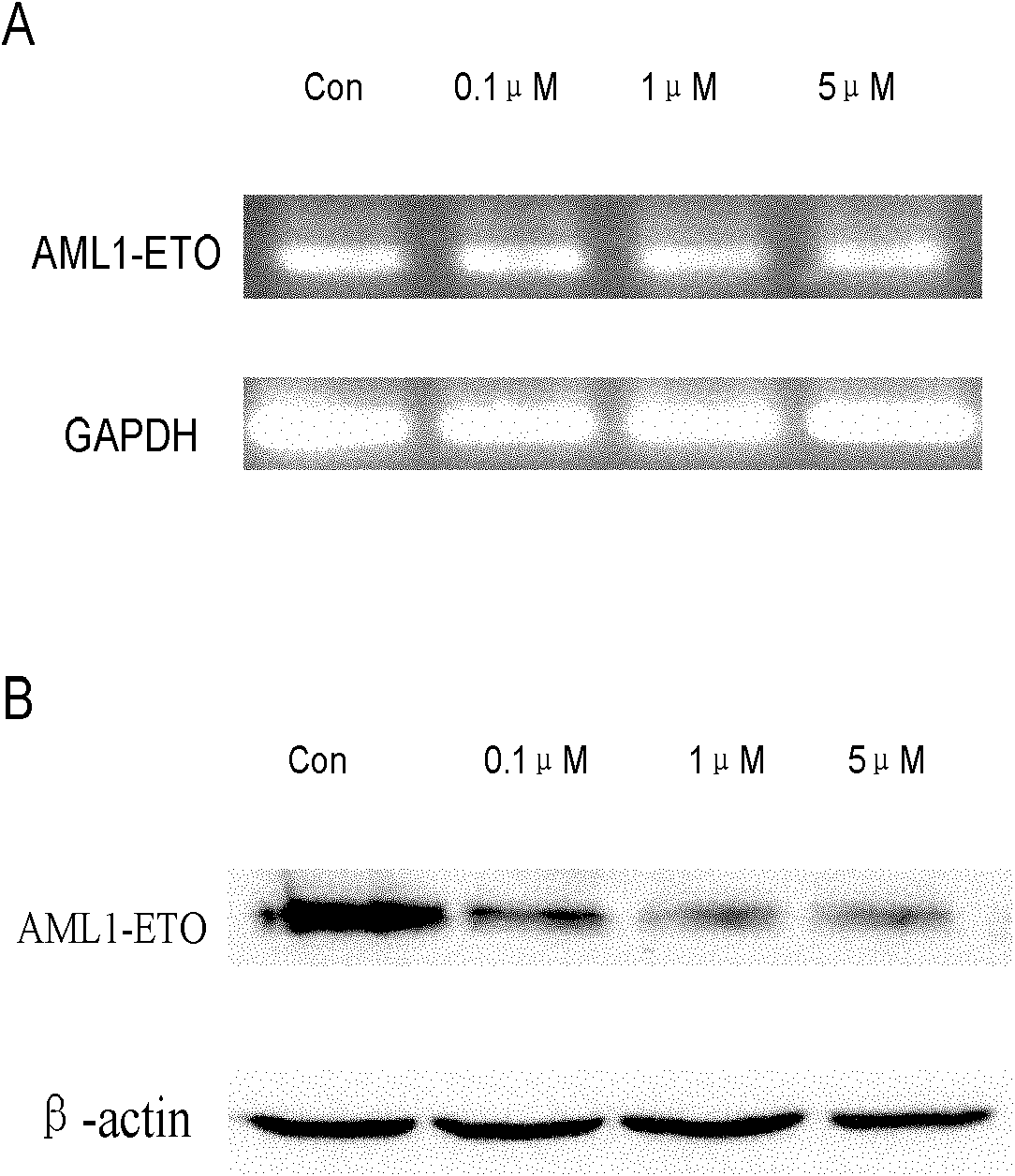
[0024] Example 2 SM1044 Inhibitory Test on Leukemia Cells
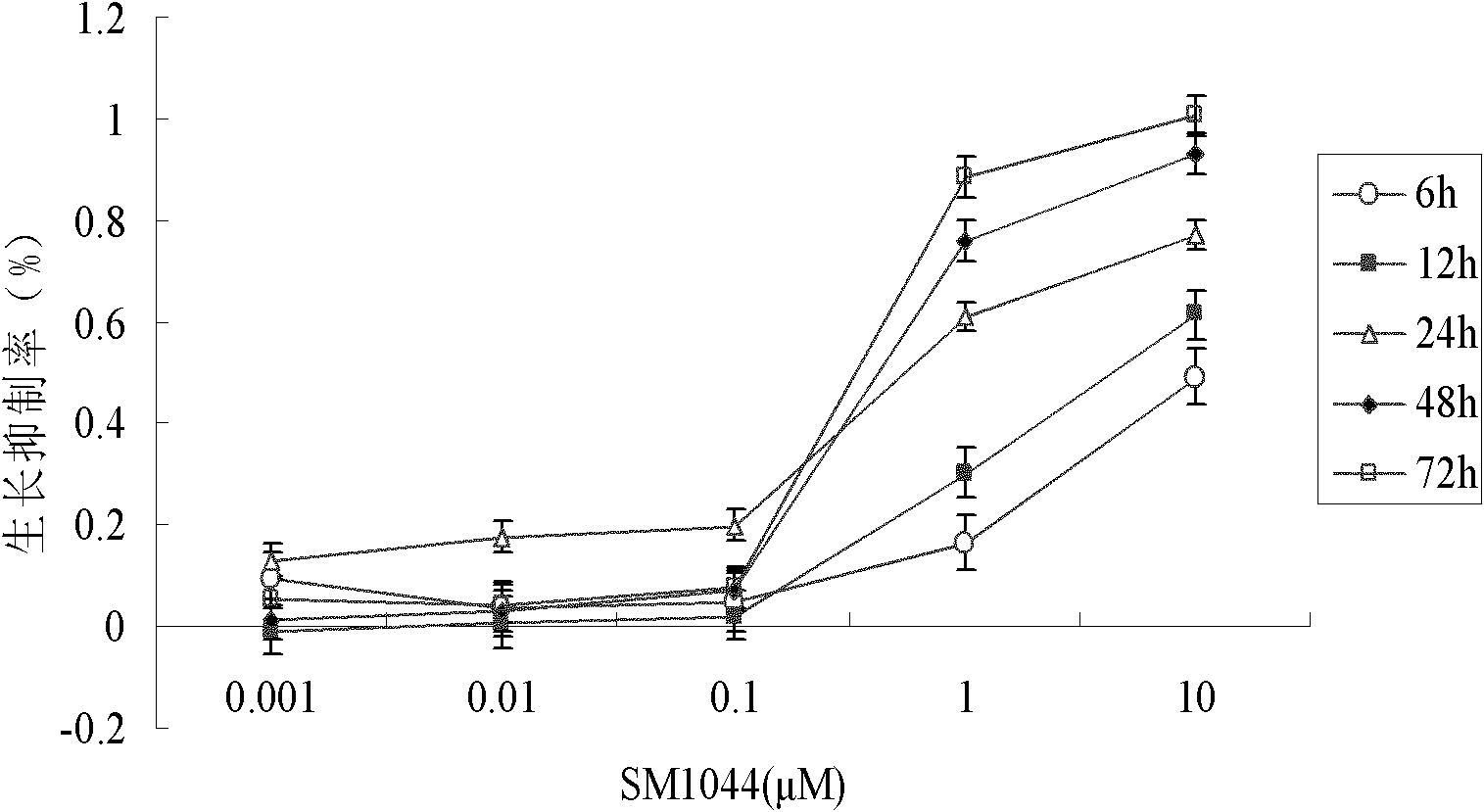
[0025] First, SM1044 was dissolved in triple distilled water at a concentration of 1 mg / ml, and then the typical acute myeloid leukemia M2b cell line Kasumi-1 cells were selected for the experiment. We will 5×10 4 Cells were suspended in 200 μl and seeded in 96-well plates. A blank group, a control group without drug addition, and a drug group with different concentrations (0.001 μM, 0.01 μM, 0.1 μM, 1 μM, 10 μM SM1044) were set up, and three parallel wells were set up for each concentration. After culturing for 24 hours, MTT (5 mg / ml) was added to continue culturing for 4 hours. Centrifuge, suck off 180 μl of supernatant, add 180 μl of DMSO to each well, and shake on a shaker for 15 minutes. Detect the absorbance value (A) at 570nm with a microplate reader, and calculate the half inhibitory concentration IC 50 . The results showed that Kasumi-1 cells were sensitive to SM1044, IC 50 It was 0.17μM, indicating tha...
Embodiment 3
[0027] Example 3 SM1044 induces Kasumi-1 cell apoptosis test
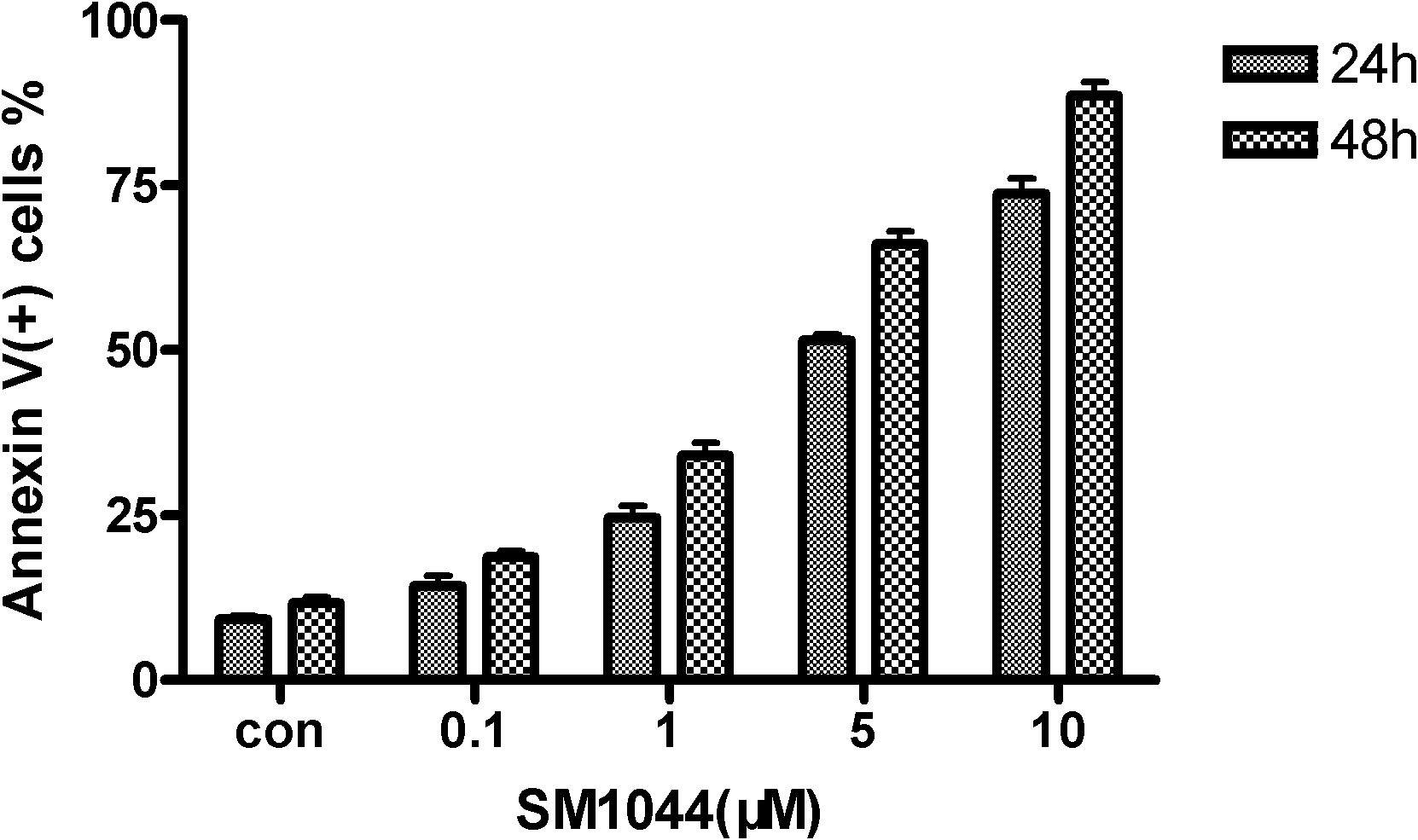
[0028] will be 5×10 5 Kasumi-1 cells were suspended in 1ml medium and seeded in 24-well plates. Set up the control group (Con), different concentration (0.1μM, 1μM, 5μM, 10μM SM1044) dosing groups, culture for 24h and 48h respectively, and collect 1×10 cells to be tested 6 First, wash twice with pre-cooled phosphate buffered saline (PBS), resuspend with 200 μl binding buffer, add 5 μl Annexin V-FITC and 5 μl propidium iodide (PI), mix gently, and place in Incubate at room temperature in the dark for 15 minutes, and detect by flow cytometry within 1 hour, the results are as follows: figure 2 As shown, the concentration of SM1044 is 0.1 μM-10 μM, and it can induce apoptosis in Kasumi-1 cells within 24-48 hours. It indicated that SM1044 could induce apoptosis of Kasumi-1 cells, and the number of apoptotic cells increased with the increase of drug concentration and treatment time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com