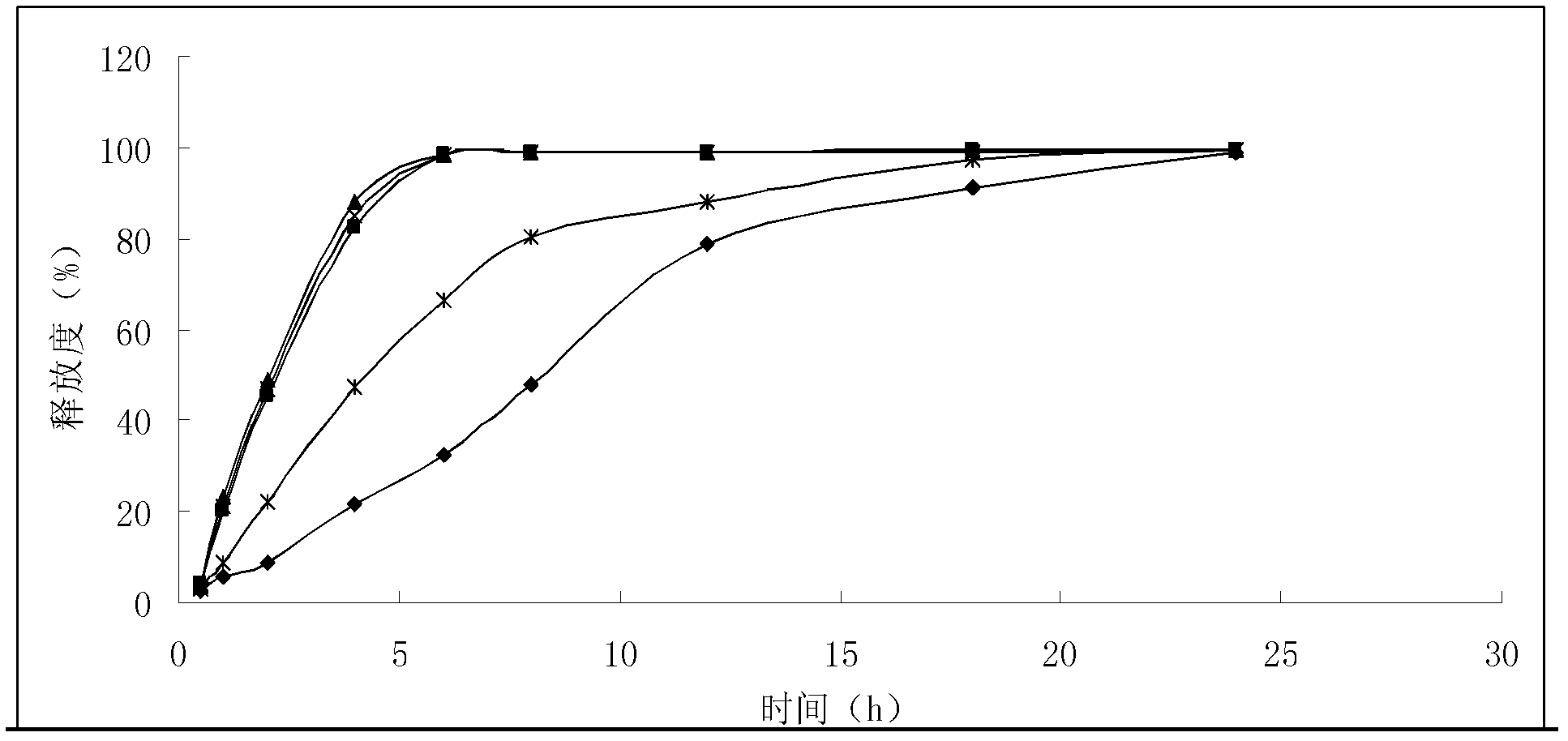
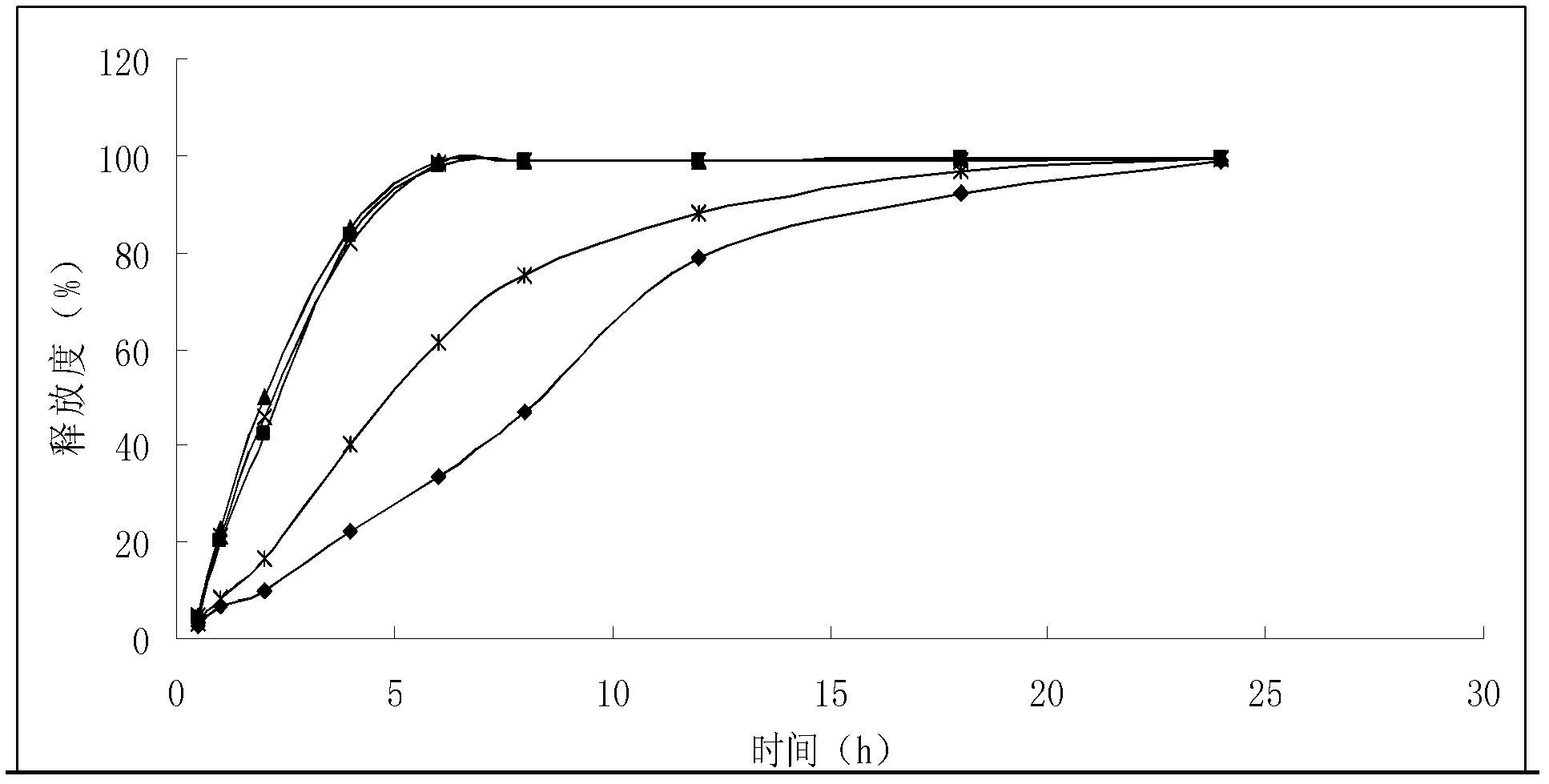
Solid preparation of bisoprolol / hydrochlorothiazide medicine composition liposome
A technology of hydrochlorothiazide and bisoprolol is applied in the field of medicine to achieve the effects of high encapsulation rate, good sustained release effect and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 Bisoprolol / Hydrochlorothiazide Liposomal Tablets
[0090]
[0091] Adopt the following production process to prepare bisoprolol / hydrochlorothiazide liposome tablet:
[0092] (1) Accurately weigh 2.5g bisoprolol fumarate, 6.25g hydrochlorothiazide, 80g hydrogenated soybean lecithin, 20g dilauroylphosphatidylcholine, 50g cholesterol, 60g Tween 60, dissolve in 1200ml with a volume ratio of 2:1 In the mixed solvent of dichloromethane and isopropanol, stir to dissolve it;
[0093] (2) Put the above solution in an eggplant-shaped bottle, remove dichloromethane and isopropanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0094] (3) Add 1200ml of phosphate buffer solution with a pH value of 7.0 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0095] (4) Filter the above solution with a 0.45 μm microporous membrane,...
Embodiment 2
[0099] Example 2 Bisoprolol / Hydrochlorothiazide Liposomal Tablets
[0100]
[0101]
[0102] Adopt the following production process to prepare bisoprolol / hydrochlorothiazide liposome tablet:
[0103] (1) Accurately weigh 5g of bisoprolol fumarate, 6.25g of hydrochlorothiazide, 80g of hydrogenated soybean lecithin, 20g of dilauroylphosphatidylcholine, 50g of cholesterol, 120g of Tween 60, and dissolve them in 1500ml of In the mixed solvent of dichloromethane and isopropanol, stir to dissolve it;
[0104] (2) Put the above solution in an eggplant-shaped bottle, remove dichloromethane and isopropanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0105] (3) Add 1500ml of phosphate buffer solution with a pH value of 7.0 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0106] (4) Filter the above solution with a 0.45 μm micropo...
Embodiment 3
[0110] Example 3 Bisoprolol / Hydrochlorothiazide Liposomal Tablets
[0111] The raw and auxiliary materials used are as follows:
[0112]
[0113]
[0114] Adopt the following production process to prepare bisoprolol / hydrochlorothiazide liposome tablet:
[0115] (1) Accurately weigh 5g of bisoprolol fumarate, 6.25g of hydrochlorothiazide, 320g of hydrogenated soybean lecithin, 80g of dilauroylphosphatidylcholine, 200g of cholesterol, 120g of Tween 60 and dissolve them in 2000ml of bismuth with a volume ratio of 2:1. In the mixed solvent of methyl chloride and isopropanol, stir to dissolve it;
[0116] (2) Put the above solution in an eggplant-shaped bottle, remove dichloromethane and isopropanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0117] (3) Add 2000ml of phosphate buffer solution with a pH value of 7.0 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com