Lentivirus expression vector of anti-p185erdB2 human mouse chimeric antibody containing F/2A sequence and construction method of lentivirus expression vector
A p185erbb2, human-mouse chimeric antibody technology, applied in botanical equipment and methods, biochemical equipment and methods, introduction of foreign genetic material using vectors, etc. Expression imbalance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
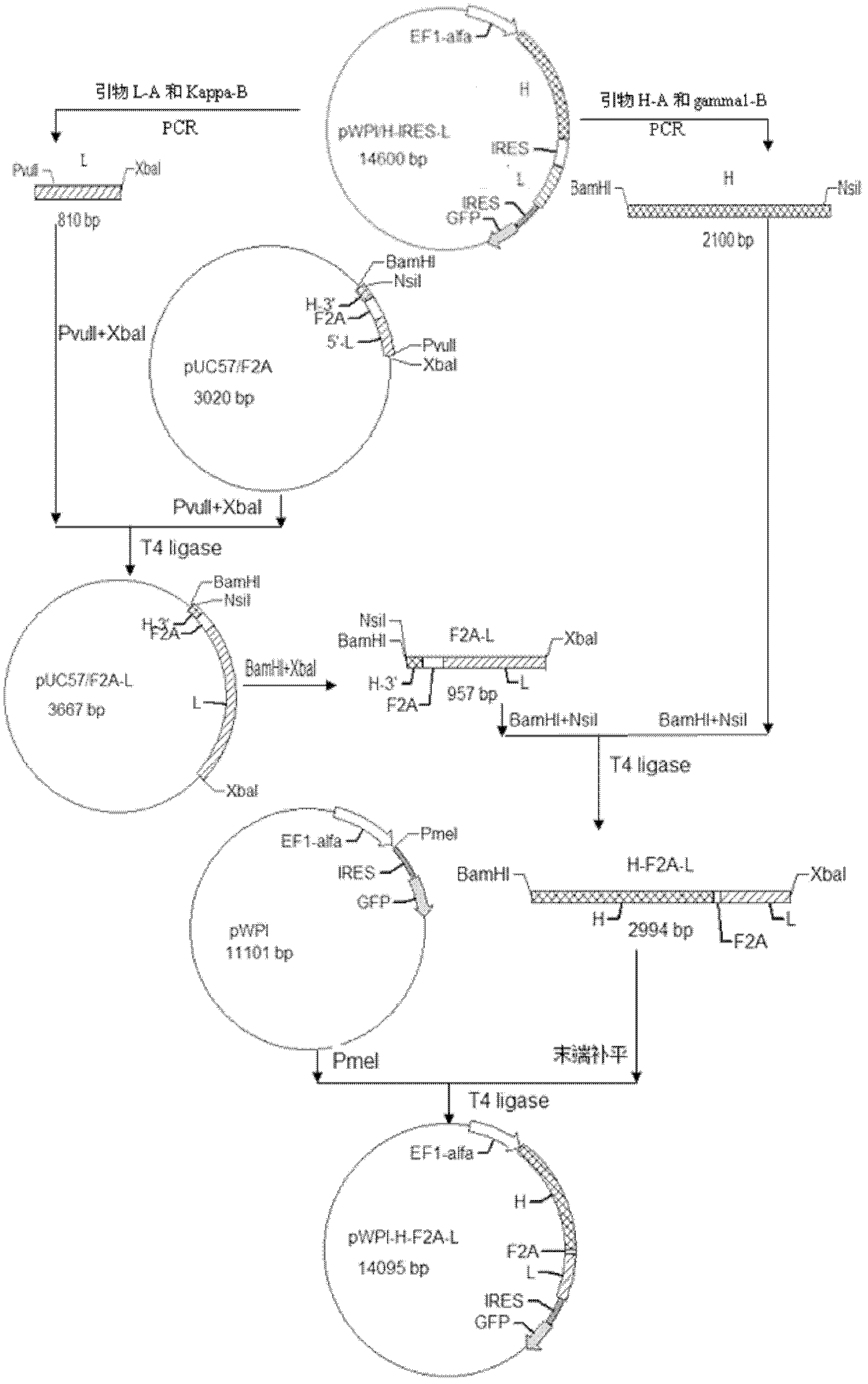
[0032] Anti-p185 with F / 2A sequence erbB2 Human-mouse chimeric antibody lentiviral expression vector and its construction method
[0033] 1 Materials and methods
[0034] 1.1 Plasmids, strains and cells
[0035] The three-plasmid lentivirus system consists of expression plasmid pWPI, structural plasmid pCMV-dR 8.74 and envelope plasmid pMD2G, donated by the Laboratory of Clinical Oncology Center, University of Ottawa, Canada. The plasmid pUC57 / F2A containing the F / 2A sequence was constructed by Shanghai Sangon Company. Transformed with Escherichia coli DH-5α containing anti-p185 erbB2 Plasmid pWPI / H-IRES-L and 293T cells of human and mouse chimeric light and heavy chain genes are preserved by Guangxi Zhuang Autonomous Region Institute of Cancer Prevention and Control.
[0036] 1.2 Tool enzymes and main reagents
[0037] Restriction enzymes BamH I, Pvu II, Nsi I, Xba I, Pme I, T4 DNA ligase, etc. were purchased from New England Biotechnology Company. Taq DNA polymerase, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com