Pentaerythritol diphosphonate dichloride ethylenediamine macromolecular flame retardant and preparation method thereof
A technology of pentaerythritol diphosphate diphosphoryl chloride ethylenediamine and pentaerythritol diphosphate diphosphoryl, which is applied in the field of "trinity" macromolecular intumescent flame retardants, can solve the problems of high-speed stirring, difficult process control, and cost Advanced problems, achieve the effect of reducing preparation cost, low reaction temperature and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
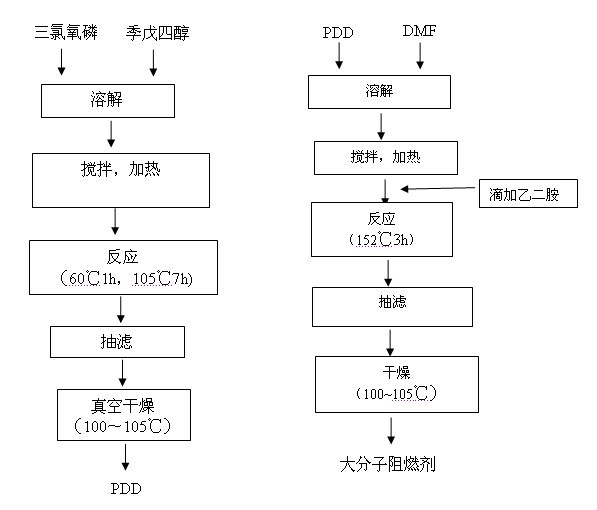
[0022] Embodiment one: preparation process sees appendix figure 1 :
[0023] [1] Add 27.2g (0.2mol) of pentaerythritol and 108.8 mL (1.2mol) of phosphorus oxychloride, heated in an oil bath, stirred, and refluxed for reaction. First react at 60°C for 1h, then at 105°C for 7h until no HCl is released. After phosphorus oxychloride was distilled off under reduced pressure, it was cooled, washed with dichloromethane, and filtered. Dry to constant weight under vacuum at 110°C to obtain pentaerythritol diphosphate diphosphoryl chloride (PDD) with a yield of about 82%.
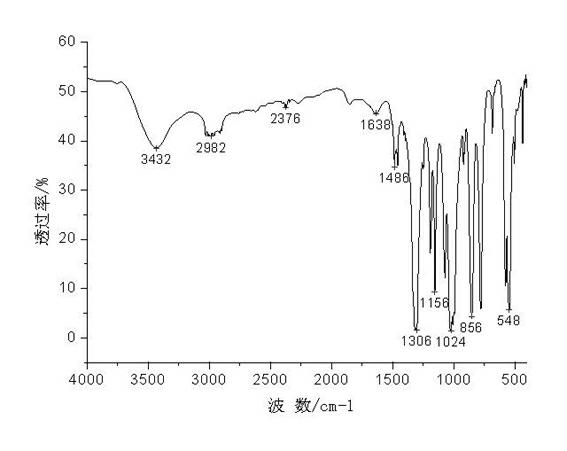
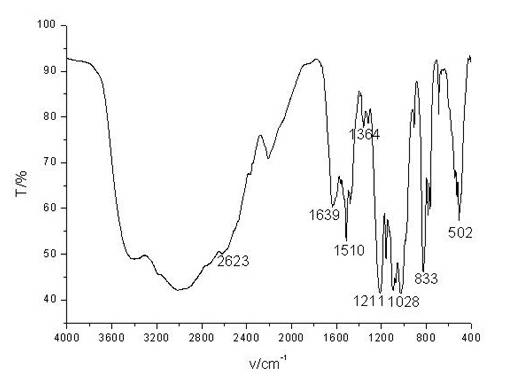
[0024] Infrared analysis was carried out on the obtained intermediate products, such as figure 2 , shows: 1306cm -1 It is the stretching vibration peak of P=O; 856cm -1 It is the stretching vibration peak of P-O; 1024 cm -1 It is the stretching vibration peak of P - O-C; 548cm -1 It is the P-Cl stretching vibration peak. This result indicated that PDD was successfully prepared.
[0025] [2] Add 2.97g of PD...
Embodiment 2
[0027] Embodiment two, test the flame retardant property of macromolecule flame retardant:
[0028] Add the macromolecular flame retardant of Example 1 to E-44 epoxy resin at a mass fraction of 5%, 10%, 20%, and 30%, stir evenly, and then add 1g of curing agent EDA to prepare a standard sample According to the GB / T2406-93 plastic oxygen index test method and the plastic vertical burning test method in UL-94-1996, the samples were tested for the limiting oxygen index (LOI) test and the plastic combustion performance test.
[0029] The results are shown in the table below. The average limiting oxygen index (LOI) of the blank epoxy resin sample is 18%. -Level 0.
[0030] Macromolecular flame retardant content (wt%) LOI (%) UL-94 0 18 V-3 5 26 V-0 10 28 V-0 20 32 V-0 30 38 V-0
[0031] The morphology of the macromolecular flame retardant flame retardant epoxy resin splines changed obviously before and after burning. The photo of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com