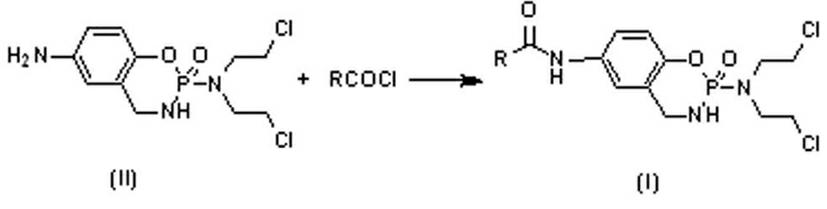
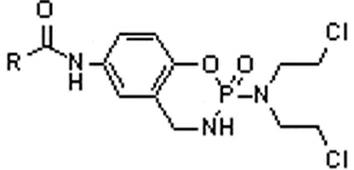
Benzocyclophosphamide phenate derivatives as well as preparation method and application thereof
A technology of benzocyclophosphamide and its derivatives, which is applied in the field of phenolic acid benzocyclophosphamide derivatives and their preparation and application, can solve the problems of bladder toxicity, prolong survival time, inhibit angiogenesis, and achieve excellent results Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
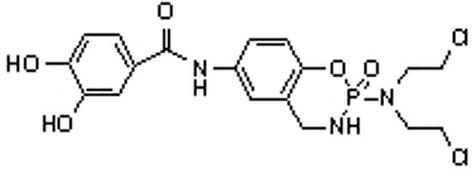
[0026] 7-(3',4'-Dihydroxybenzamido)-2-[bis(2-chloroethyl)amino]-1,3,2-benzoxaphos-2-oxide (1) Preparation of:
[0027] (1) structure see image 3 , under the protection of nitrogen, add 1.54g (0.01mol) 3.4-dihydroxybenzoic acid and 20mL dioxane to the dry reactor, add 2.2mL (0.03mol) of thionyl chloride dropwise, and react at 95-100°C for 3 After 1 hour, add 3.89 g (0.012 mol) of intermediate (II) in 20 mL of dioxane solution, and continue the reaction at 95-100°C for 5-6 hours. The solvent was recovered under reduced pressure, separated and purified by silica gel column chromatography to obtain 7-(3',4'-dihydroxybenzamido)-2-[bis(2-chloroethyl)amino]-1,3,2- Benzoxaphos-2-oxide (1). 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 2.78 (m, 4H, CH 2 ), 3.21 (m, 1H, NH), 3.52-3.92 (m, 6H, CH 2 ), 5.35 (s, 2H, OH), 6.82-7.45 (m, 6H, Ar-H), 9.13 (s, 1H, Ar-NH). Anal Calcd for C 18 h 20 Cl 2 N 3 o 5 P : C, 46.97; H, 4.38; N, 9.13. Found: 46.90; H, 4.37; N, 9.14.
Embodiment 2
[0029] 7-(3',4'-dimethoxybenzamido)-2-[bis(2-chloroethyl)amino]-1,3,2-benzoxaphos-2-oxide ( 2) Preparation:
[0030] (2) structure see Figure 4 , replace 3.4-dihydroxybenzoic acid with 3,4-dimethoxybenzoic acid in Example 1, and others are the same as Example 1 to obtain 7-(3',4'-dimethoxybenzamido) -2-[bis(2-chloroethyl)amino]-1,3,2-benzoxaphos-2-oxide (2). 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 2.80 (m, 4H, CH 2 ), 3.24 (m, 1H, NH), 3.52-3.92 (m, 12H, CH 2 , OCH 3 ), 6.80-7.65 (m, 6H, Ar-H), 9.12 (s, 1H, Ar-NH). Anal Calcd for C 20 h 24 Cl 2 N 3 o 5 P : C, 49.19; H, 4.95; N, 8.61. Found: 49.12; H, 4.95; N, 8.60.
Embodiment 3
[0032] 7-(3',4'-diacetoxybenzamido)-2-[bis(2-chloroethyl)amino]-1,3,2-benzoxaphos-2-oxide ( 3) Preparation:
[0033] (3) structure see Figure 5 , replace 3.4-dihydroxybenzoic acid with 3,4-diacetoxybenzoic acid in Example 1, and the others are the same as Example 1 to obtain 7-(3',4'-diacetoxybenzamido) -2-[bis(2-chloroethyl)amino]-1,3,2-benzoxaphos-2-oxide (3). 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 2.25 (s, 6H, CH 3 ), 2.84(m, 4H, CH 2 ), 3.23 (m, 1H, NH), 3.54-3.91 (m, 6H, CH 2 ), 6.80-7.81 (m, 6H, Ar-H), 9.15 (s, 1H, Ar-NH). Anal Calcd for C 22 h 24 Cl 2 N 3 o 7 P : C, 48.54; H, 4.44; N, 7.72. Found: 48.52; H, 4.44; N, 7.73.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com