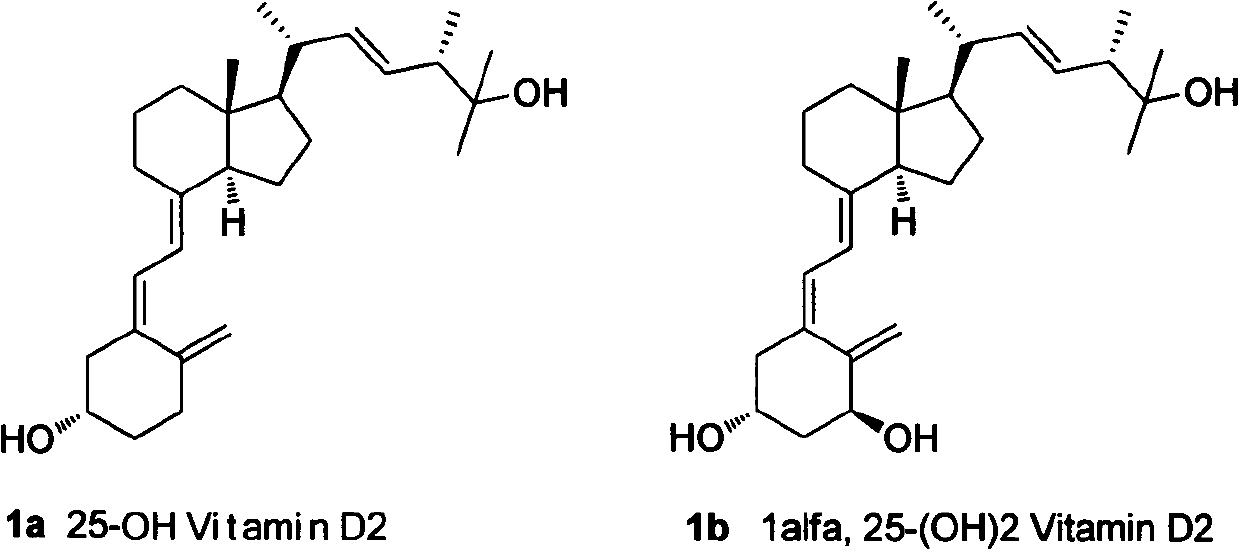
Method for preparing intermediates for synthesizing 25-hydroxyvitamin D2 and 1 alpha, 25-dihydroxyvitamin D2
A technology of dihydroxyvitamin and hydroxyvitamin, which is applied in the field of preparation of two organic compounds, can solve the problems of low yield, limitation, difficulty in synthesis and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
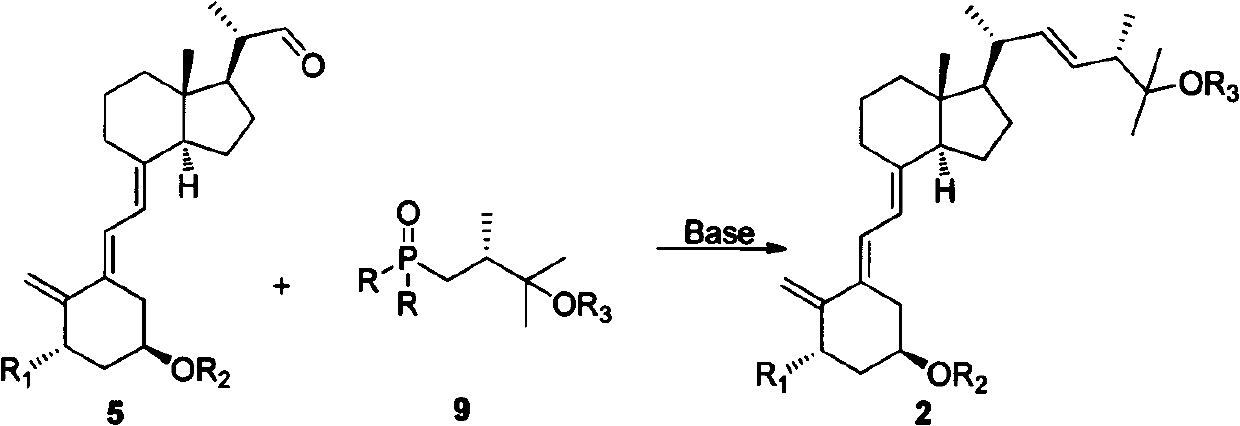
[0026] Example 1: Preparation methods of compounds 2a-1, 2b-1, 3a-1 and 3b-1. where R is phenyl, R 2 is tert-butyldimethylsilyl, R 3 For trimethylsilyl; R in 2a-1 and 3a-1 1 is a hydrogen atom, R in 2b-1 and 3b-1 1 Be methoxymethoxy, its reaction formula is as follows respectively (formula 5):
[0027]
[0028]
[0029] Formula 5
[0030] Dissolve 600 mg of compound 9-1 in 10 mL of anhydrous tetrahydrofuran, cool to minus 78 degrees Celsius under nitrogen protection, and then slowly drop 1.0 mL of 1.6 mol / L butyllithium in tetrahydrofuran solution into it. The reaction was stirred for 30 minutes, and then 500 mg of 5a-1 tetrahydrofuran solution was slowly dropped into it below -40 degrees Celsius, and the reaction was continued for 2 hours with stirring. Naturally raised to room temperature, quenched with saturated ammonium chloride, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, concentrated, and the residue was subjected t...
Embodiment 2
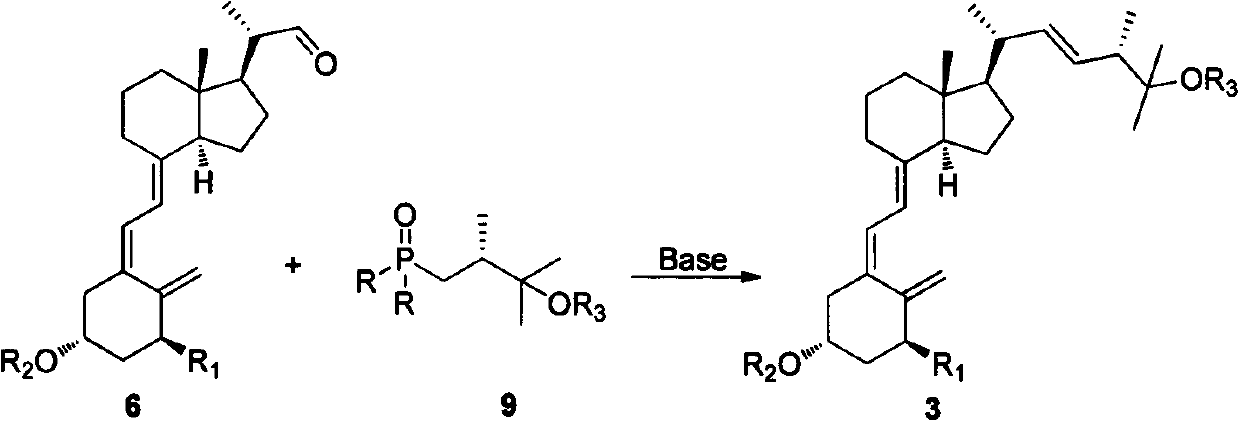
[0032] Example 2: Preparation methods of compounds 2a-2, 2b-2, 3a-2 and 3b-2. Where R is p-tolyl, R 2 is methoxymethyl, R 3 For 2-tetrahydropyranyl; R in 2a-2 and 3a-2 1 is a hydrogen atom, R in 2b-2 and 3b-2 1 Be triethylsilyloxy group, its reaction formula is as follows respectively (formula 6):
[0033]
[0034] Formula 6
[0035]Dissolve 800 mg of compound 9-2 in 10 mL of anhydrous tetrahydrofuran, cool to minus 78 degrees Celsius under nitrogen protection, and then slowly drop 2.5 mL of 1.0 mol / L tetrahydrofuran solution of tert-butyllithium into it. Continue to stir and react for 30 minutes, and then slowly drop 640 mg of 5a-2 tetrahydrofuran solution into it below -70 degrees Celsius, and continue to stir and react for 2 hours. Naturally raised to room temperature, quenched with saturated ammonium chloride, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, concentrated, and the residue was subjected to silica gel column chr...
Embodiment 3
[0037] Example 3: Preparation methods of compounds 2a-3, 2b-3, 3a-3 and 3b-3. where R is methoxy, R 2 is 2-tetrahydrofuranyl, R 3 For methoxymethyl; R in 2a-3 and 3a-3 1 is a hydrogen atom, R in 2b-3 and 3b-3 1 Be benzoyloxy group, its reaction formula is as follows respectively (formula 7):
[0038]
[0039] Formula 7
[0040] Take 100 mg of sodium hydride and add it to 10 mL of anhydrous n-hexane, cool it to zero degrees Celsius under the protection of nitrogen, and then slowly add 800 mg of compound 9-3 in n-hexane solution dropwise therein. Then, 700mg of 5a-3 n-hexane solution was slowly dropped into it at 0°C, and the stirring reaction was continued for 5h. Naturally raised to room temperature, quenched with water, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, concentrated, and the residue was subjected to silica gel column chromatography to obtain 0.60 g of 2a-3 respectively.
[0041] In the same way, 700mg of 5b-3 was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com