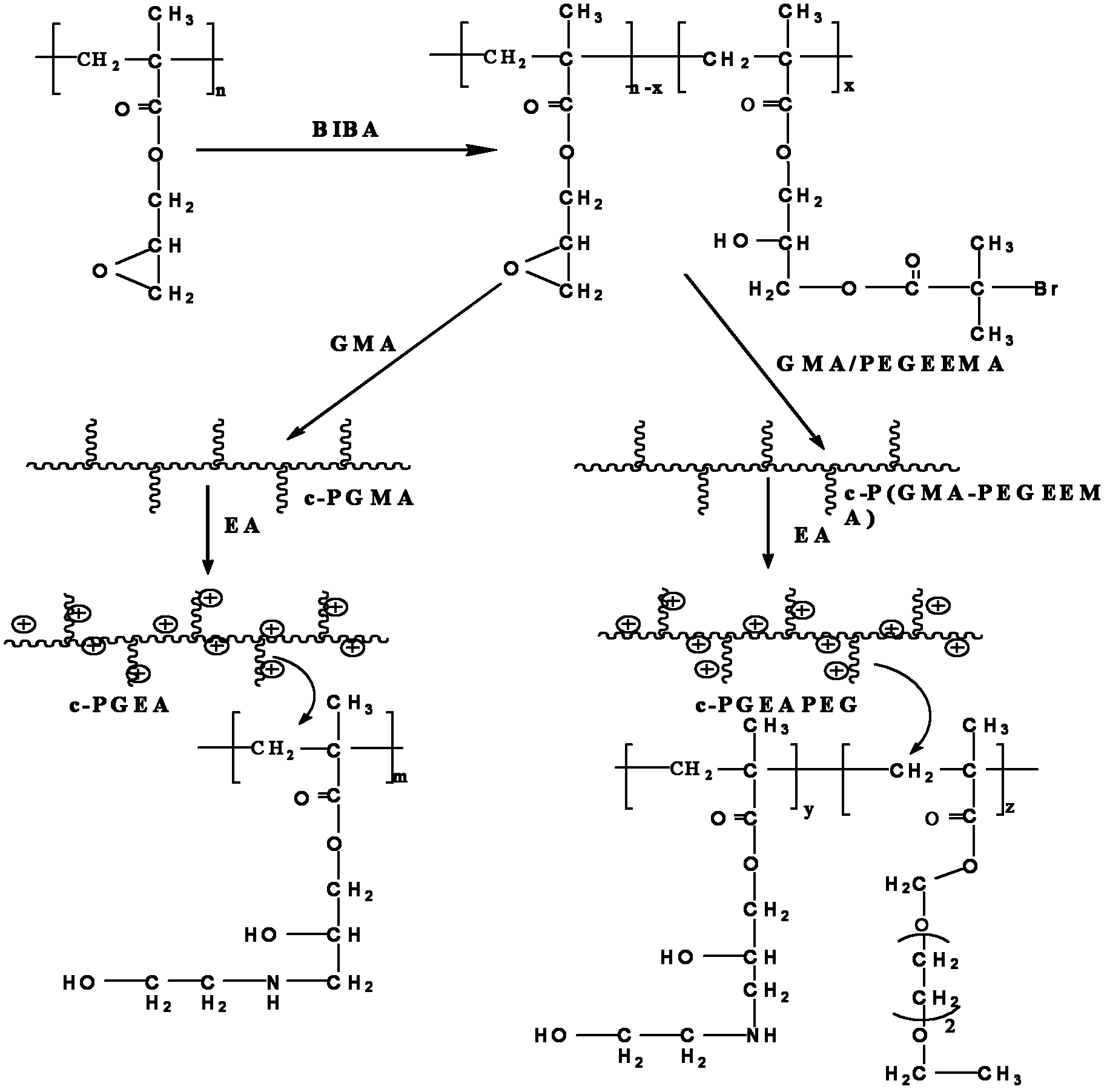
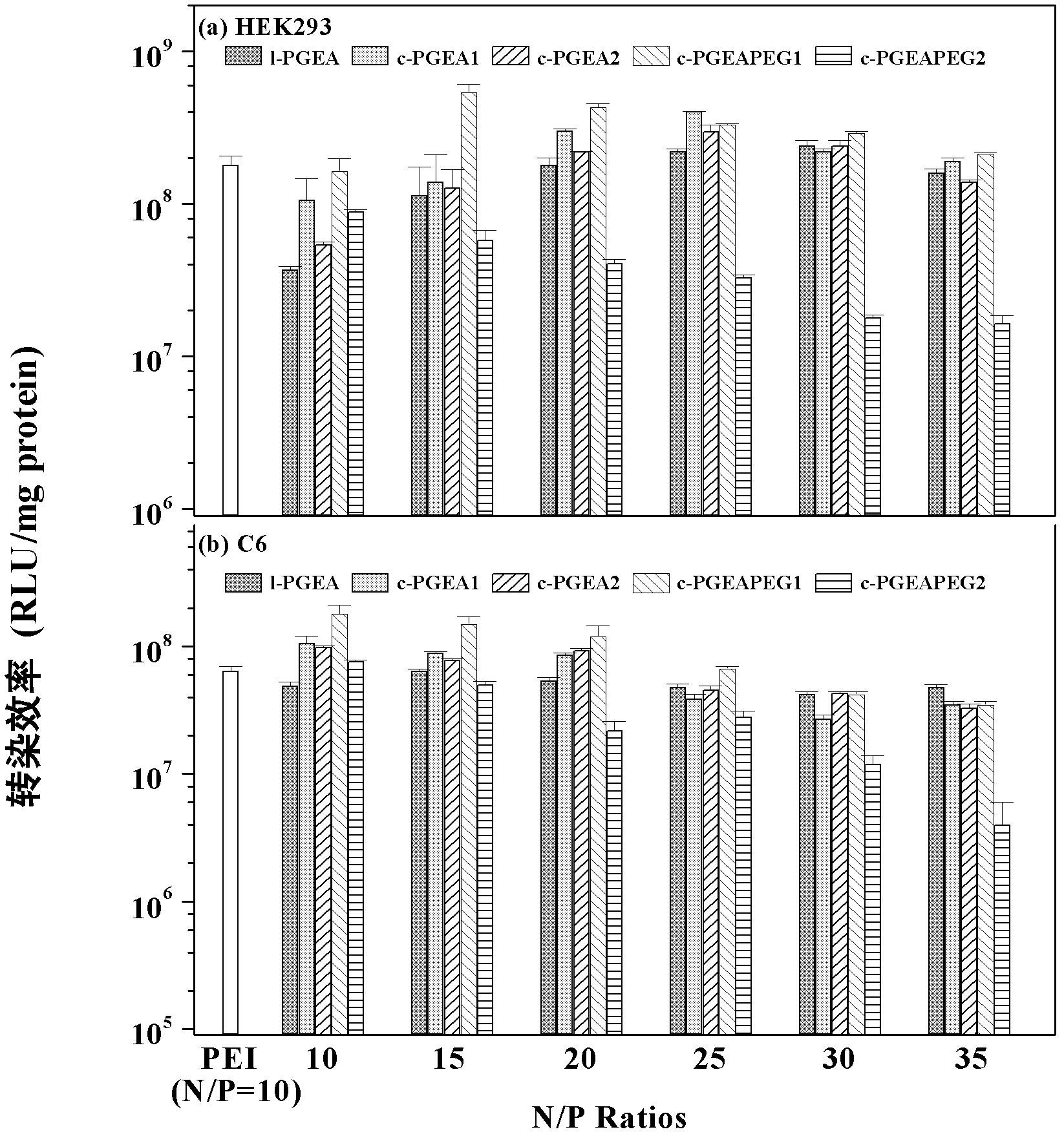
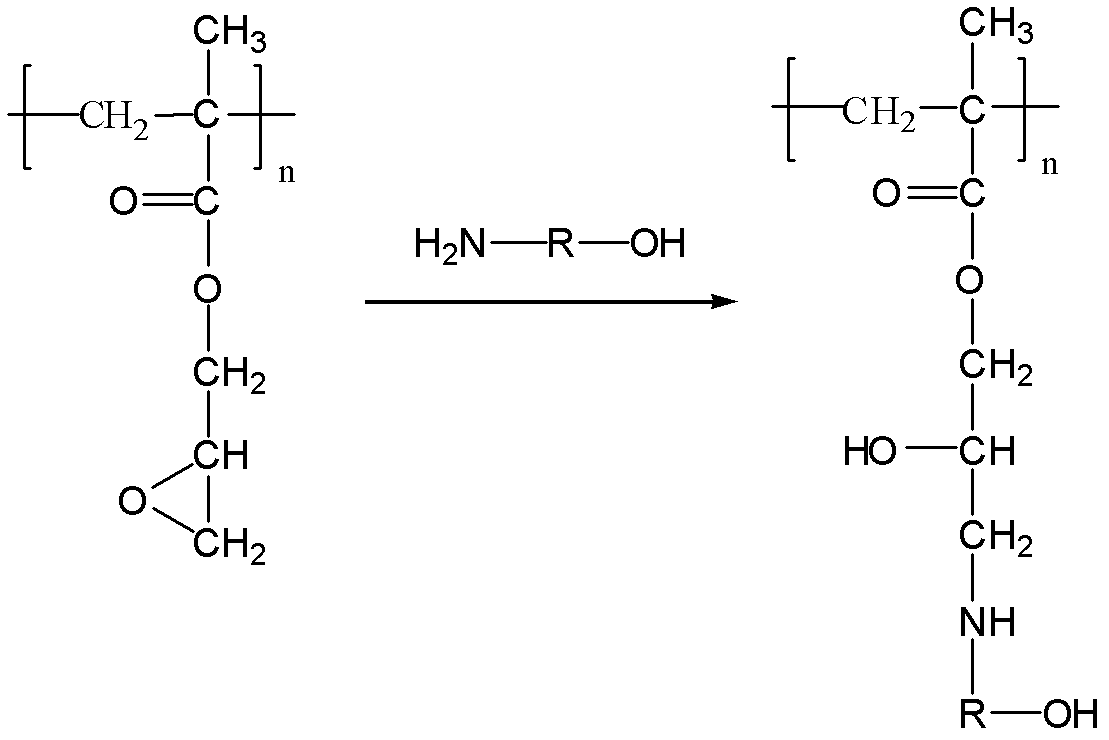High-performance cationic gene vectors with PGMA (polyglycidyl methacrylate) serving as framework constructed by ATRP (atom transfer radical polymerization) method
A gene carrier and cation technology, applied in the field of cationic gene carriers, can solve the problems of different vector transfection efficiency, different protonation ability, etc., and achieve the effects of simple use method, easy regulation and good storage stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Continuously react under nitrogen protection at 50℃, add 12g GMA (glycidyl methacrylate) into a small flask, then add 10g THF (tetrahydrofuran), 120mg pentamethyldiethylenetriamine, 213.6mg PMDETA, and finally Add 86.4mg CuBr to initiate active controllable free radical polymerization; after 3h, open the stopper and accelerate the stirring for 10min, fully contact with air to stop the reaction. The polymerized product is repeatedly precipitated with methanol until the morphology becomes solid, and then placed in a vacuum drying box to remove the methanol , The linear PGMA is obtained, the number average molecular weight (Mn) of the polymer is 580000 g / mol, and the PDI (Mw / Mn) is 1.23.
[0035] 2) Continuous reaction under nitrogen protection at 50℃, add 0.3g of linear PGMA obtained in step 1) to 5.6g of THF to dissolve, and then add 3g of ethanolamine (or use 2-amino-1-propanol, 3-amino-1- Propanol, NN-dimethylethylenediamine, or 0.15g ethanolamine and 0.15g NN-dimethyl...
Embodiment 2
[0037] 1) Continue the reaction under nitrogen protection at 37°C. Take 1.5 g of the linear PGMA obtained in step 1) of Example 1 in a flask and dissolve it in 10 g of THF, add 0.36 g of BIBA (2-Bromoisobutyric acid), and react for 24 hours. , Ether precipitation, vacuum drying to obtain PGMA-Br (PGMA / BIBA is 5:1, one of every 6 GMA segments on the molecular chain is connected with Br).
[0038] 2) Continuous reaction under nitrogen protection at 50℃, add 0.3g of PGMA-Br obtained in step 1) above to 5g THF to dissolve, then add 4gGMA, 82mg HMTETA, and finally 33.5mg CuBr to initiate active controllable free radicals Polymerization, after 4 hours of reaction, open the stopper and accelerate the stirring for 10 minutes, and fully contact with the air to stop the reaction; the polymerization product is repeatedly precipitated with methanol until the product becomes solid, and the comb-shaped PGMA is obtained after the methanol is removed in a vacuum drying cabinet.
[0039] 3) Continu...
Embodiment 3
[0041] 1) Continuous reaction at 37°C under nitrogen protection, take 2.5 g of the linear PGMA obtained in step 1) of Example 1 in a flask and dissolve it in 10 g of THF, add 0.37 g of BIBA (2-Bromoisobutyric acid) and react for 24 hours. Ether precipitation, vacuum drying to obtain PGMA-Br (PGMA / BIBA is 8:1, one of every 8 GMA segments on the molecular chain is connected with Br).
[0042] 2) Continuous reaction under nitrogen protection at 50℃, add 0.3g of PGMA-Br obtained in step 1) above to 5g THF to dissolve, then add 4gGMA, 82mg HMTETA, and finally 33.5mg CuBr to initiate active controllable free radicals Polymerization, after 4 hours of reaction, open the stopper and accelerate the stirring for 10 minutes, and fully contact with the air to stop the reaction; the polymerization product is repeatedly precipitated with methanol until the product becomes solid, and the comb-shaped PGMA is obtained after the methanol is removed in a vacuum drying cabinet.
[0043] 3) Continuous r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com