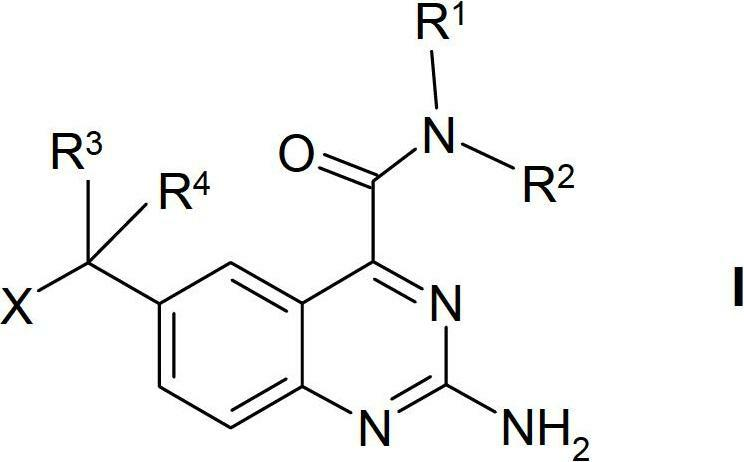
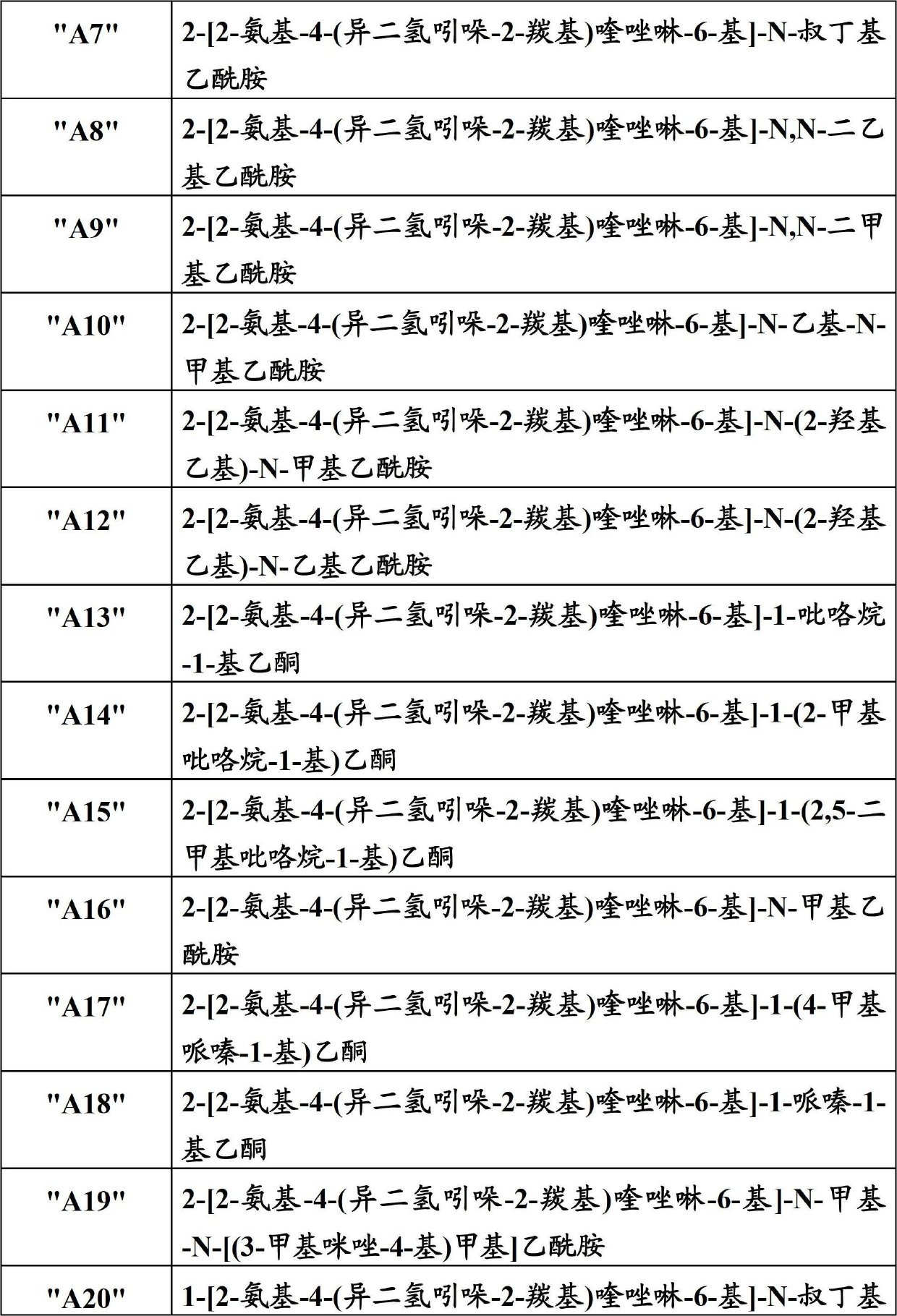
Quinazoline derivatives
A technology of compounds and mixtures, applied in the treatment of diseases in which HSP90 plays a role, those compounds for the preparation of drugs, and the treatment of HSP90-induced diseases can solve problems such as misregulation of molecular functions and physiological functions, loss of wild-type functions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] Preparation of ethyl 2-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]acetate ("A1") and 2-[2-amino-4-(isodihydro Indoline-2-carbonyl)quinazolin-6-yl]acetic acid ("A2")
[0336] step 1: Ethyl 2-[4-[[(2Z / E)-2-hydroxyiminoacetyl]amino]phenylacetate
[0337]
[0338] Dissolve 18g of chloral hydrate in 100ml of water, add 27g of Na 2 SO 4 , and the mixture was stirred at 23° C. for 10 minutes. A solution of 20 g of ethyl 4-aminophenylacetate hydrochloride in 100 ml of water was added to the solution. A solution of 19 g of hydroxylamine hydrochloride in 50 ml of water was added to the resulting suspension, and the mixture was stirred at 60° C. for 90 minutes. The mixture was then cooled, during which a precipitate settled. It was filtered off, washed with water and dried under vacuum at 40°C. The resulting mixture was used in subsequent reactions without further purification.
[0339] Yield: 11.2 g (2-[4-[[(2Z / E)-2-hydroxyiminoacetyl]amino]phenylacetate ethyl...
Embodiment 2
[0370] Preparation of 2-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]acetamide ("A3")
[0371]
[0372] 100 mg of "A1" was dissolved in 40 ml of methanol, and 135 mg of magnesium nitride was added. The mixture was stirred at 80°C for 12 hours, cooled, diluted with 10 ml of water and adjusted to pH 2 with 25% hydrochloric acid. The resulting precipitate was filtered off and purified by column chromatography. Yield: 18mg (20%) of 2-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]acetamide; LC-MS retention time: 0.74min;
[0373] 1 H NMR (500MHz, DMSO-d 6 / TFA -d 1 ):δ[ppm]7.98(dd,J=8.7,1.8,1H),7.93(d,J=1.4,1H),7.73(d,J=8.5,1H),7.48(d,J=7.3,1H ), 7.34 (dt, J=17.9, 6.9, 2H), 7.25 (d, J=7.3, 1H), 5.05 (s, 2H), 4.80 (s, 2H), 3.58 (s, 2H).
[0374] The compound 1-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]-5,5-difluoropentanamide ("A4") was obtained analogously
[0375]
[0376] Yield: 15mg (16%); LC-MS retention time: 1.64min;
[0377] 1 H NMR (500MHz, ...
Embodiment 3
[0383] Preparation of 2-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]-N-ethylacetamide ("A6")
[0384]
[0385] Dissolve 50 mg of "A2" in 2 ml ethylamine and microwave at a maximum of 120 °C (CEM )60 minutes. The mixture was evaporated to dryness in vacuo, the residue was dissolved in DMSO and purified by column chromatography. Yield: 8 mg (16%) of 2-[2-amino-4-(isoindoline-2-carbonyl)quinazolin-6-yl]-N-ethylacetamide;
[0386] LC-MS retention time: 1.53min;
[0387] 1 H NMR (500MHz, DMSO-d 6 / TFA -d 1 ):δ[ppm]7.99(dd,J=8.6,1.8,1H),7.95(d,J=1.2,1H),7.75(d,J=8.5,1H),7.48(d,J=7.2,1H ),7.34(dt,J=18.6,6.9,2H),7.25(d,J=7.3,1H),5.07(s,2H),4.81(s,2H),3.59(s,2H),3.07(q ,J=7.2,2H),0.99(t,J=7.2,3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com