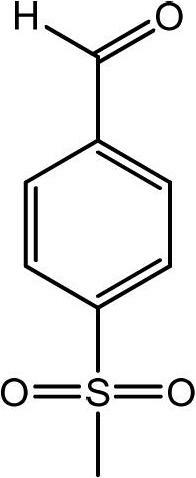
Preparation method of p-methylsulfonyl benzaldehyde
A technology for p-methylsulfonyl and p-chlorobenzaldehyde is applied in the preparation of thiamphenicol pharmaceutical intermediates and in the field of preparation of pharmaceutical intermediates. The effect of short production cycle, short reaction route and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
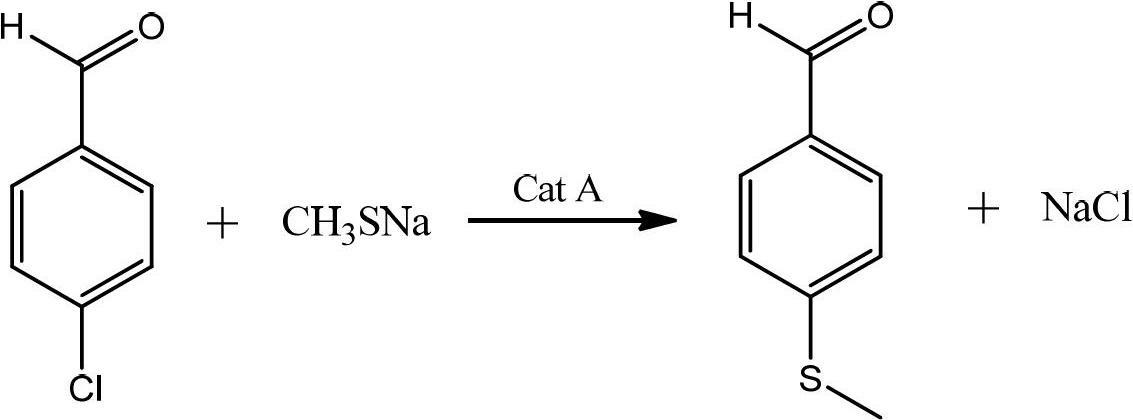
[0032] According to p-chlorobenzaldehyde: sodium methylmercaptide: the mol ratio of tetrabutylammonium bromide is 1: 1.1: 0.025 and carries out following reaction:
[0033] Under stirring conditions, add 20% by mass concentration of sodium methyl mercaptide aqueous solution, tetrabutylammonium bromide, and 250g p-chlorobenzaldehyde to the reaction kettle, control the temperature at 45-50°C for reaction, and monitor the reaction by TLC until p-chlorobenzaldehyde The reaction of benzaldehyde was complete, and the layers were separated after standing to obtain 290 g of yellow oil in the lower layer, and the yield of crude p-methylthiobenzaldehyde was 107.2%.
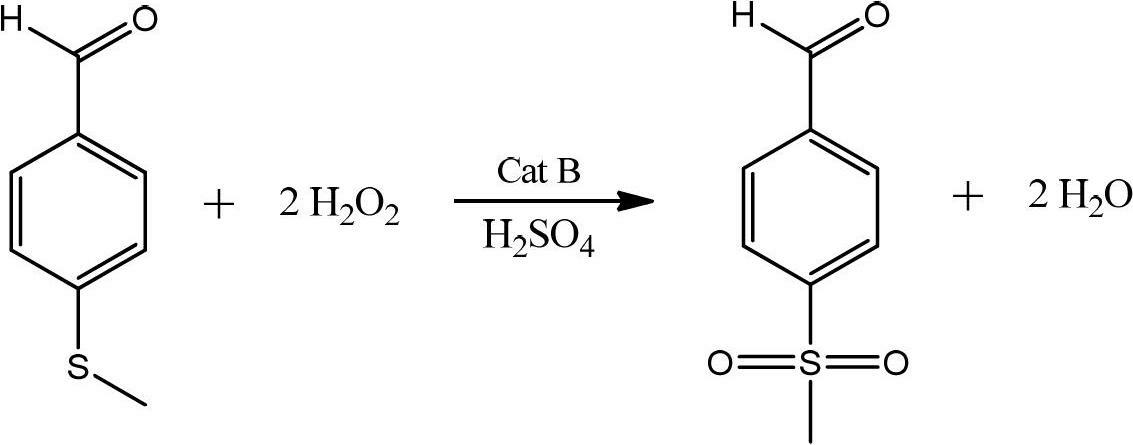
[0034] According to p-methylthiobenzaldehyde: hydrogen peroxide: sulfuric acid: the mol ratio of sodium tungstate is 1: 2.5: 0.02: 0.015 and proceeds as follows:
[0035] Under stirring, add 50% concentration of hydrogen peroxide, sodium tungstate and sulfuric acid into the reaction kettle, raise the temperature to 40-50°C,...
Embodiment 2
[0039] According to p-chlorobenzaldehyde: sodium methylmercaptide: tetrabutylammonium chloride mol ratio is 1: 2: 0.2 and carry out following operations:
[0040] Add 250g of p-chlorobenzaldehyde, 30% aqueous solution of sodium methyl mercaptide and tetrabutylammonium chloride into the reaction kettle under stirring, raise the temperature to 55-60°C for reaction, monitor by TLC, until the reaction of p-chlorobenzaldehyde is complete, let stand Separate layers to obtain 296 g of yellow oil in the lower layer, and the yield of crude p-methylthiobenzaldehyde is 109.4%.
[0041] According to p-methylthiobenzaldehyde: hydrogen peroxide: sulfuric acid: manganese sulfate mol ratio is 1: 2.5: 0.02: 0.05 and carries out following operations:
[0042] Add 20% concentration of hydrogen peroxide, manganese sulfate and concentrated sulfuric acid into the reaction kettle, stir and heat up to 40-45°C, add the whole batch of crude p-methylthiobenzaldehyde dropwise, control the reaction temper...
Embodiment 3
[0044] According to p-chlorobenzaldehyde: sodium methylmercaptide: tetrabutylammonium iodide mol ratio is 1: 1.5: 0.1 and carry out following operation:
[0045] Add 250g of p-chlorobenzaldehyde, 15% aqueous solution of sodium methyl mercaptide and tetrabutylammonium chloride into the reaction kettle under stirring, raise the temperature to 50-55°C for reaction, monitor by TLC, until the reaction of p-chlorobenzaldehyde is complete, let stand Separate layers to obtain 288 g of yellow oil in the lower layer, and the yield of crude p-methylthiobenzaldehyde is 106.5%.
[0046] According to p-methylthiobenzaldehyde: hydrogen peroxide: sulfuric acid: manganese sulfate mol ratio is 1: 4: 0.1: 0.1 carries out following operation:
[0047] Add 30% concentration of hydrogen peroxide, manganese sulfate and concentrated sulfuric acid into the reaction kettle, stir and heat up to 40-45°C, add the whole batch of crude p-methylthiobenzaldehyde dropwise, control the reaction temperature at 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com