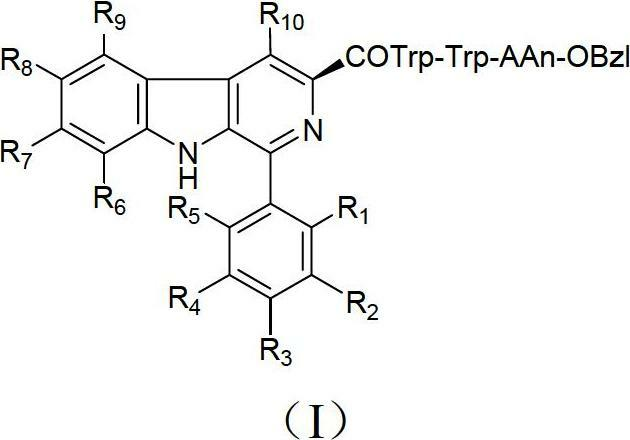
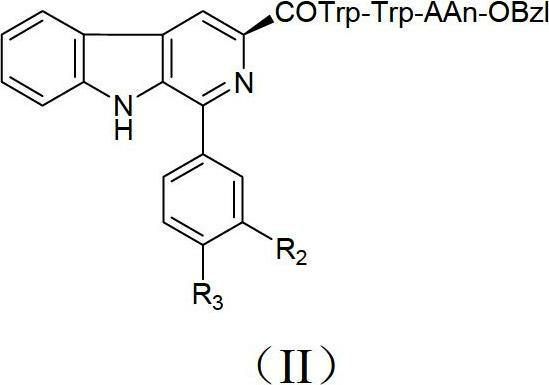
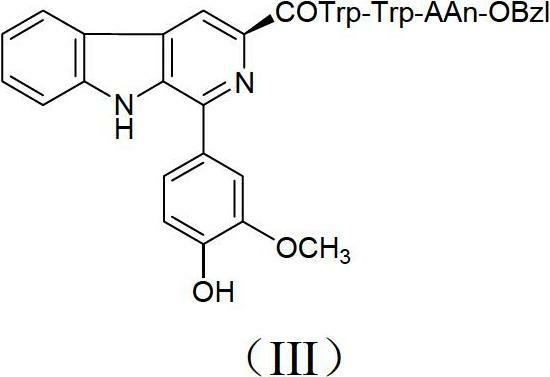
Beta-carboline derivatives, and preparation method and application thereof
A compound and pharmaceutical technology, applied in the field of beta-carboline derivatives, can solve the problems of limited effectiveness, poor bioavailability of anticancer drugs, and poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0046] Preparation Example 11- Preparation of (4-hydroxy-3-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid
[0047]
[0048] Weigh 8g (39.2mmol) of L-tryptophan and 10g (65.8mmol) of 3-methoxy-4-hydroxybenzaldehyde into a 250ml eggplant bottle, add 150ml of glacial acetic acid, stir, heat to reflux at 70°C, and react first It was turbid, and clarified after a period of reaction, and finally a large amount of white solids were precipitated. Monitored by TLC plate, the raw material spots disappeared, stopped the reaction and cooled down to room temperature, under ice bath, added ammonia water to adjust the pH to 5, a large amount of white solid precipitated, filtered. The title compound of 3 was obtained as a white solid (3.5 g, 10.4 mmol, 27%).
[0049] [α] 25 D -20.36(c 0.1,MeOH);
[0050] IR (cm -1 ,KBr,neat):3325,1716,763;
[0051] 1 HNMR (300MHz, DMSO-d 6 ):δ10.79(1H,s,NH),7.48(1H,d,J=7.8Hz,Ar-H),7.27(1H,d,J=7.8Hz,Ar-H),7.06(3H,m ,Ar-H),6.75(1H,d...
preparation example 2
[0055] Preparation Example 21- Preparation of (4-hydroxy-3-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester
[0056]
[0057] Gradually add 8ml of thionyl chloride dropwise to 100ml of methanol under ice bath. After dropping, stir for 20 minutes. Then add 3.5g (10.36mmol) 1-(4-hydroxy-3-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylate acid. The reaction mixture was heated at reflux at 60° C. and stirred for 6 hours, and TLC detection showed that the starting material disappeared. The reaction mixture was concentrated under reduced pressure. The residue was crystallized from methanol / ether to give the title compound 4 (1 g, 2.57 mmol, 25%) as a white solid.
[0058] [α] 25 D +186.3(c 0.1,MeOH);
[0059] IR (cm -1 ,KBr,neat):3097,1747,740;
[0060] 1 HNMR (300MHz, DMSO-d 6 ):δ10.80(1H,s,NH),9.58(1H,s,NH),7.54(1H,d,J=7.5Hz,Ar-H),7.31(1H,d,J=7.8Hz,Ar -H),7.10(3H,m,Ar-H),6.94(2H,s,Ar-H),5.84(1H,s,CH),4.74(1H,m,CH),3.85(3H,s, OCH...
preparation example 3
[0064] Preparation Example 31- Preparation of (4-hydroxy-3-methoxyphenyl)-β-carboline-3-carboxylic acid methyl ester
[0065]
[0066] Weigh 1g (2.57mmol) 1-(4-hydroxy-3-methoxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester and 2.86g (25.8 mmol) SeO 2 , add to 250ml eggplant bottle, add 100ml dioxane, heat and reflux at 60°C for 4 hours, TLC board detects that the raw material spots disappear, stop the reaction and drop to room temperature, spin dry under reduced pressure, add 100ml distilled water, under ice bath, Add ammonia to adjust the pH to 9, filter, and rinse the filter cake with distilled water. Drying gave the title compound 5 as a brown solid (500 mg, 1.44 mmol, 56%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com