Method for preparing L10-FePt Nano-particles through water bath alternate reduction
A technology of l10-fept and nanoparticles, applied in the field of alternate reduction of water bath to prepare L10-FePt nanoparticles, can solve the problems of carbonyl iron being highly toxic and unsuitable for large-scale use, and achieve the effect of reducing the ordering temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
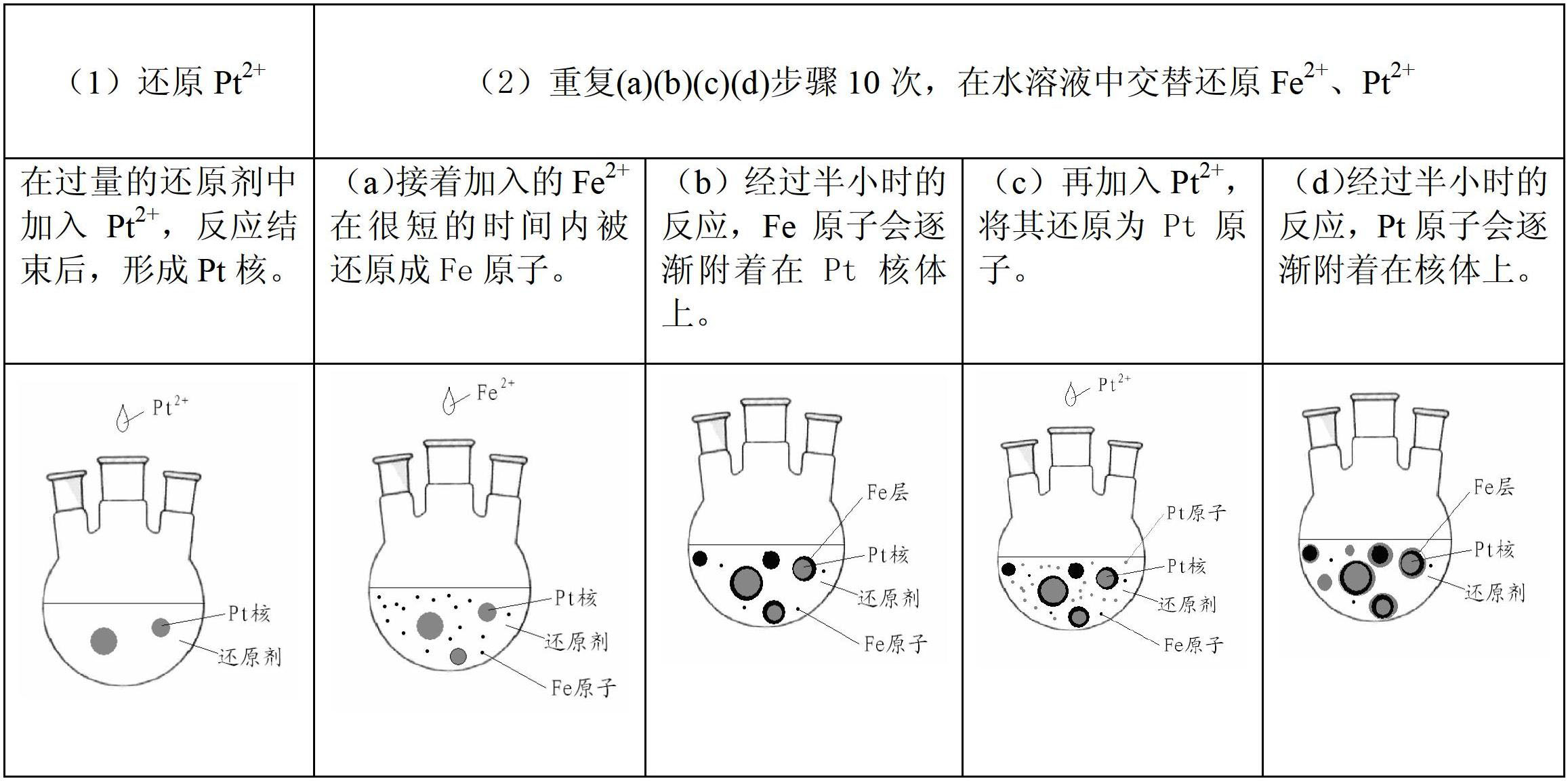
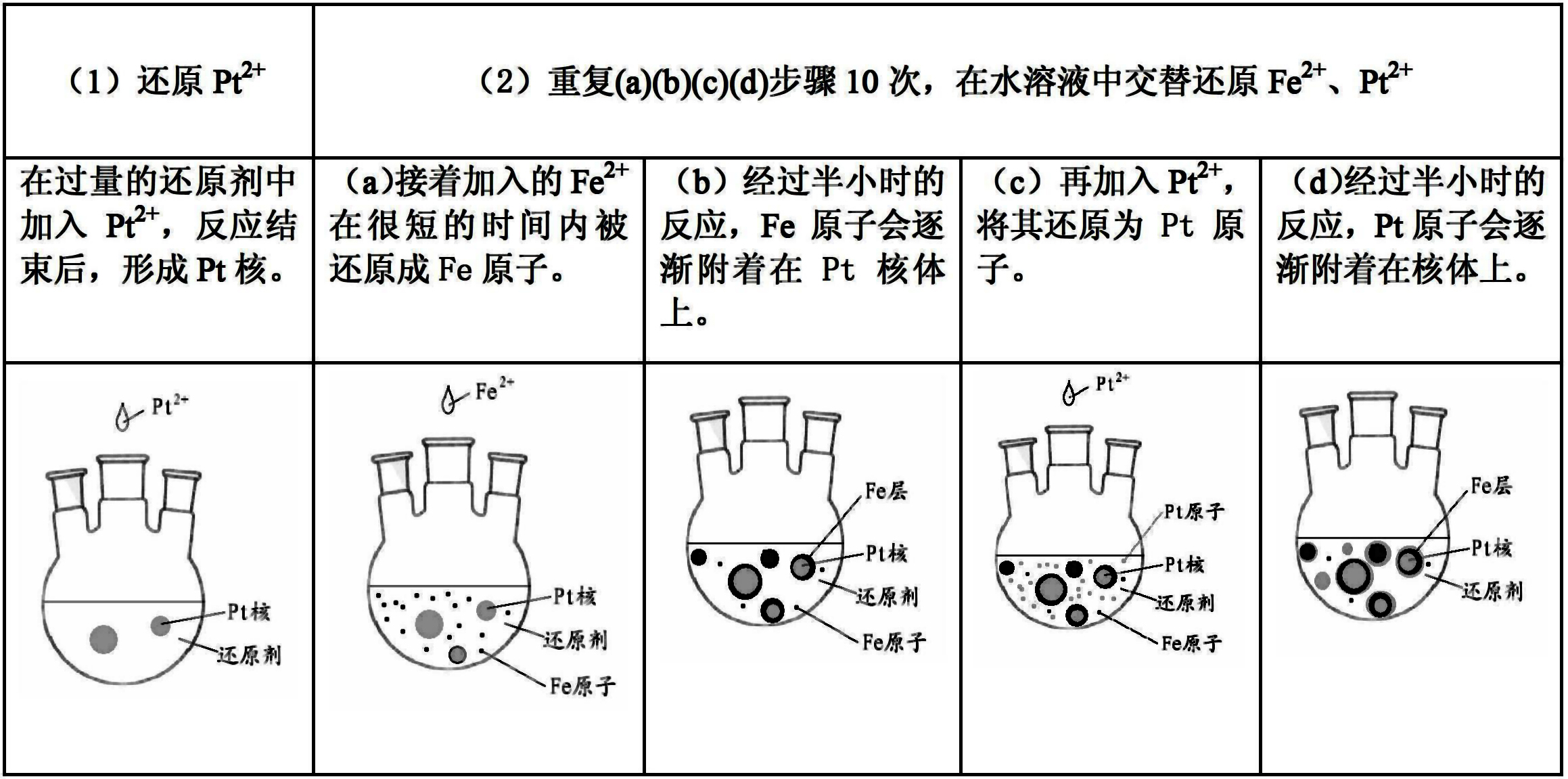
[0016] 1. With the sample: the H 2 PtCl 6 ·6H 2 O(n Pt mol) is soluble in water, the volume of water (liter) is related to n pt (mole) ratio is 25:1, dubbed into aqueous solution; FeCl 2 4H 2 O(n Fe mol) is soluble in water, the volume of water (liter) is related to n Fe (mole) ratio is 25:1, made into an aqueous solution, weighed CTAB n Fe +n Pt mol.
[0017] 2. Experimental steps
[0018] ①Put CTAB into a three-necked flask to make an aqueous solution. The ratio of the volume of water (liter) to the molar amount of CTAB is 25:1. Heat the three-neck flask to 70°C in a collector-type constant temperature heating magnetic stirrer, keep stirring and argon until the end of the experiment.
[0019] ② Set the molar weight as n Fe +n Pt The hydrazine hydrate was added to the three-necked flask, and then the Pt 2+ One-tenth of the solution was dripped into the three-neck flask very slowly (dropping time was 10 minutes) and evenly. After the dropwise addition was compl...
preparation example 2
[0025] 1. Sample preparation is the same as Example 1
[0026] 2. Experimental steps
[0027] ① Put CTAB(n Fe +n Pt mol) into a three-necked flask to form an aqueous solution, in which the ratio of the volume of water (liter) to the molar amount of CTAB is 25:1. Heat the three-neck flask to 70°C in a collector-type constant temperature heating magnetic stirrer, keep stirring and pass argon protection gas until the end of the experiment.
[0028] ② put n Fe +n Pt Moles of hydrazine hydrate were added to the three-necked flask, and then the Fe 2+ One-tenth of the solution was dripped into the three-necked flask very slowly (dropping time was 10 minutes) and evenly, and reacted for 30 minutes.
[0029] ③Inject Pt with an infusion set needle 2+ One-tenth of the solution was slowly and evenly dropped into the three-necked flask (dropping time was 10 minutes), and reacted for 30 minutes.
[0030] ④ Repeat steps ② and ③ 10 times, adding FeCl alternately 2 4H 2 O solution a...
preparation example 3
[0033] 1. Sample preparation is the same as Example 1
[0034] 2. Experimental steps
[0035]
x1
x2
x3
x4
x5
x6
x7
x8
x9
x10
Fe 2+
0.025V
0.042V
0.058V
0.072V
0.092V
0.108V
0.125V
0.142V
0.158V
0.175V
Pt 2+
0.025V
0.042V
0.058V
0.072V
0.092V
0.108V
0.125V
0.142V
0158V
0.175V
[0036] ① Put CTAB(n Fe +n Pt mol) into a three-necked flask to form an aqueous solution, wherein the ratio of the volume of water (liter) to the molar amount of CTAB is 25:1. Heat the three-neck flask to 70°C in a collector-type constant temperature heating magnetic stirrer, keep stirring and pass argon protection gas until the end of the experiment.
[0037] ② put n Fe +n Pt Mole of hydrazine hydrate was added to the three-necked flask, and then x1 ml FeCl 2 4H 2 O solution is dropped ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com