Expression system
A technology of expression system and expression box, applied in the field of expression system, can solve problems such as undesirable and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
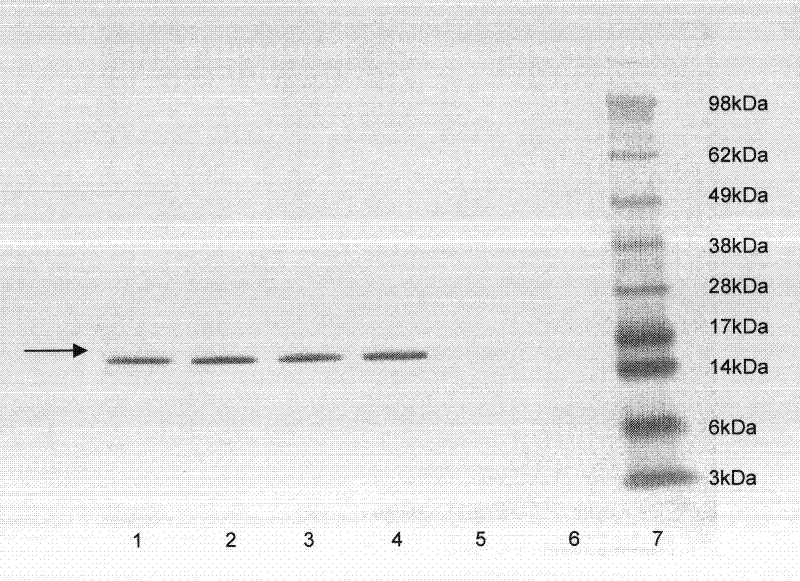
[0193] Remove the CLD032 vial from the -80°C freezer and allow it to thaw. Inoculate 10 μl of the thawed glycerol stock solution into 5 ml of LB culture medium supplemented with tetracycline (10 μg / ml) and glucose (1 g / L) (LB, 5 g / L yeast extract (Oxoid), 10 g / L tryptone (Oxoid) and 5g / L sodium chloride). Incubate for 16 hours at 37°C in an orbital shaker. 500 μl of this culture was then used to inoculate two 250 ml Erlenmeyer flasks containing 50 ml of LB broth (composition as described above). The Erlenmeyer flasks were incubated in an orbital shaker at 37°C, 200 rpm. Monitor growth until OD 600 = 0.5-0.7. At this time, one Erlenmeyer flask was induced with IPTG (isopropyl-β-D-1-thiogalactopyranoside) at a final concentration of 0.05 mM, while the second Erlenmeyer flask was not induced and kept for monitoring basal expression. Incubation was continued under the conditions described above, during which time samples were taken for growth measurements, hTNF[alpha] accumul...
Embodiment 4
[0199] Remove the CLD018 vials from the -80 °C freezer and allow them to thaw. Inoculate 10 μl of the thawed glycerol stock solution into 5 ml of LB culture medium supplemented with tetracycline (10 μg / ml) and glucose (1 g / L) (LB, 5 g / L yeast extract (Oxoid), 10 g / L tryptone (Oxoid) and 5g / L sodium chloride). The seed cultures were incubated for 16 hours at 37°C in an orbital shaker. 500 [mu]l of seed culture was then used to inoculate a 250 ml Erlenmeyer flask containing 50 ml of LB broth (composition as described above). The Erlenmeyer flasks were incubated in an orbital shaker at 37°C, 200 rpm. Monitor growth until OD 600 = 0.5-0.7. At this point the Erlenmeyer flasks were induced with IPTG (isopropyl-β-D-1-thiogalactopyranoside) at final concentrations of 0.05 mM and 1 mM. The Erlenmeyer flask was also left without induction, and the incubation of the Erlenmeyer flask was continued under the above conditions, during which time samples were taken for growth measurement...
Embodiment 5
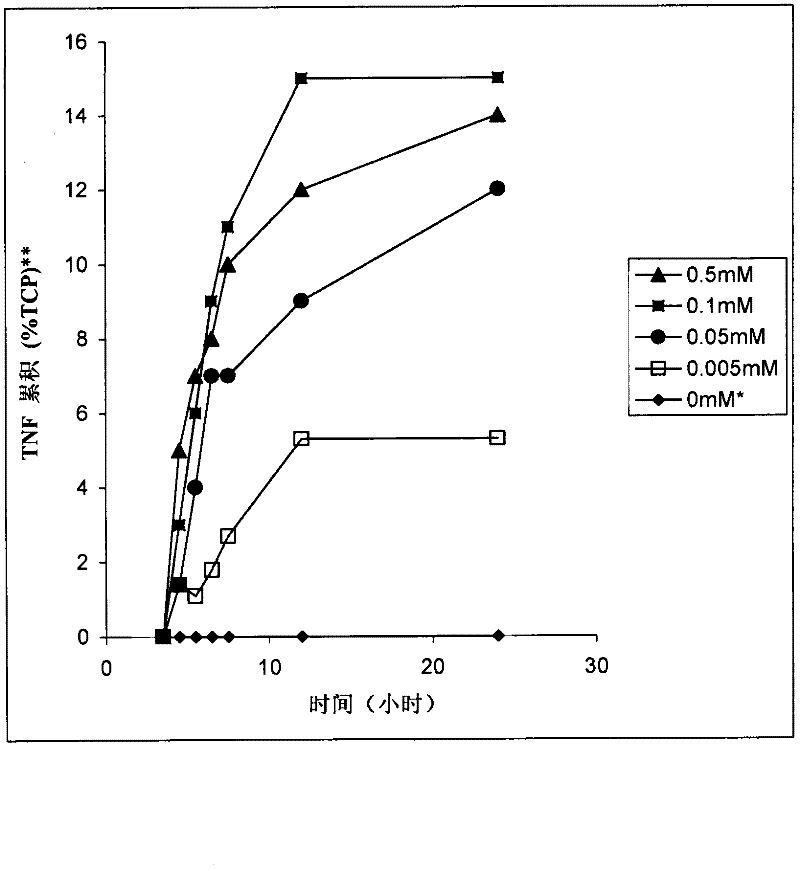
[0204] Remove the CLD026 vials from the -80 °C freezer and allow them to thaw. Inoculate 10 μl of the thawed glycerol stock solution into 5 ml of LB culture medium supplemented with tetracycline (10 μg / ml) and glucose (1 g / L) (LB, 5 g / L yeast extract (Oxoid), 10 g / L tryptone (Oxoid) and 5g / L sodium chloride). Incubate for 16 hours at 37°C in an orbital shaker. 500 [mu]l of this culture was then used to inoculate a 250 ml Erlenmeyer flask containing 50 ml of LB broth (composition as described above). The Erlenmeyer flasks were incubated in an orbital shaker at 37°C, 200 rpm. Monitor growth until OD 600 = 0.5-0.7. At this point the Erlenmeyer flasks were induced with IPTG (isopropyl-β-D-1-thiogalactopyranoside) at final concentrations of 0.05 mM and 0.005 mM. The Erlenmeyer flask was also left without induction, and the incubation was continued under the above-mentioned conditions, during which samples were taken for growth measurement and hTNFα accumulation in bacterial ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com