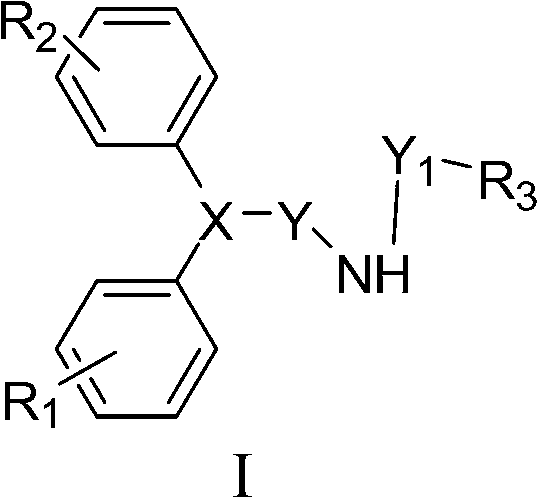
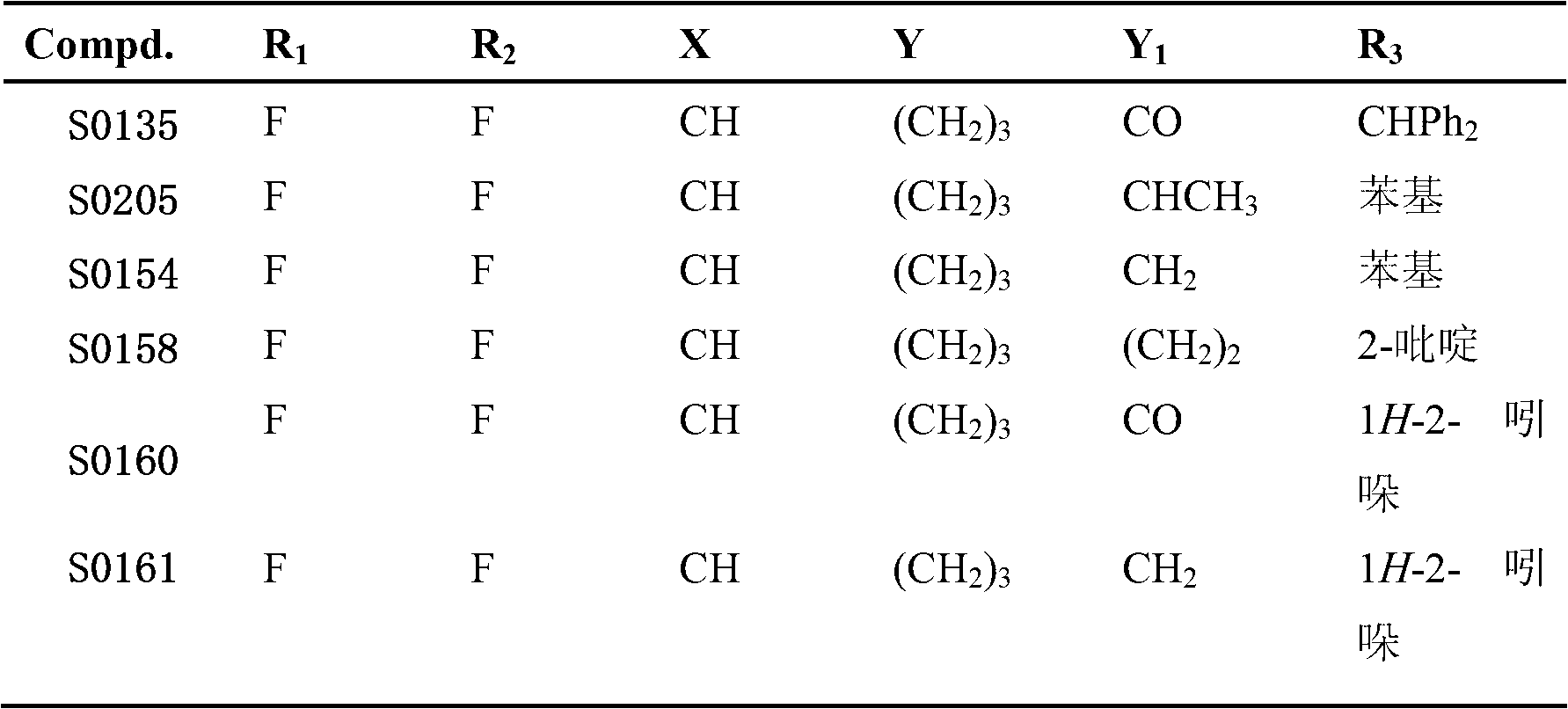
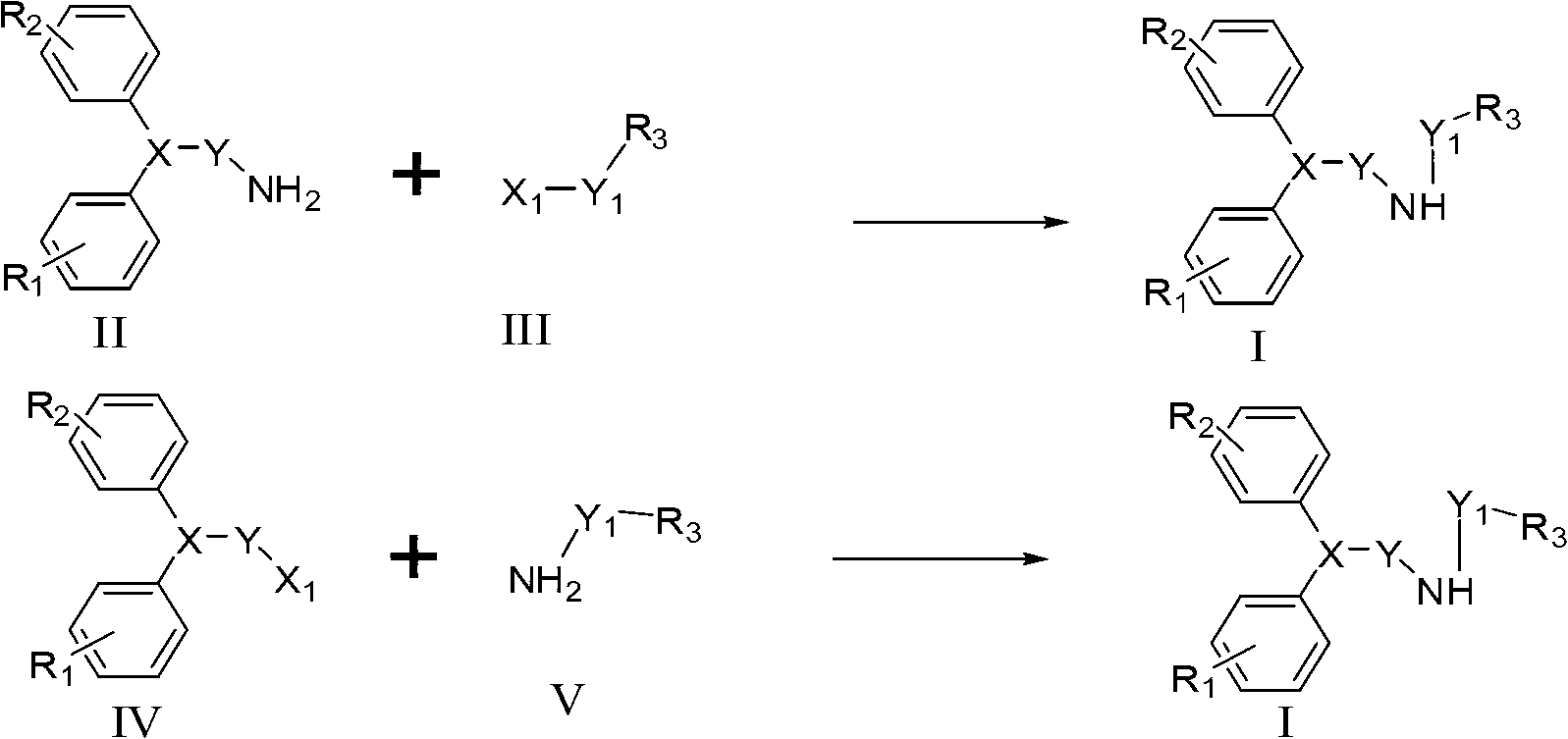
Anti-tumour compound, pharmaceutically accepted salts as well as preparation method and application thereof
A compound and amine compound technology, applied in the field of new compounds in the field of medicinal chemistry, can solve the problems of high toxicity and side effects, low killing activity, etc., and achieve the effects of convenient industrial production, broad application prospects and simple preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of (S)-N-1-(1-phenylethyl)-1-(4,4-bis(4-fluorophenyl))butylamine (Compound I-1, S0205):
[0035]
[0036] 4,4-bis(4-fluorophenyl)-1-chlorobutane (compound II-1, 0.50g, 1.78mmol) and (S)-1-phenylethylamine (compound III-1, S0205 , 0.53g, 3.58mmol) into DMSO (8mL) in a 25mL single-port reaction vial with magnetic stirring, and stirred at 80°C for 10 hours. The reactant was transferred to a 250mL separatory funnel, diluted with 100mL of ethyl acetate, and then diluted with 30mL of 5% Na 2 CO 3 Wash twice with aqueous solution, then wash twice with 50 mL of water, and finally wash once with saturated 50 mL of NaCl aqueous solution. Then the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain (S)-N-1-(1-phenylethyl)-1-(4,4-bis(4-fluorophenyl) )) Butylamine (compound 1-1, S0205, 0.59 g, 91% yield). 1 H-NMR (CDCl 3 ,400MHz):7.33~7.21(m,5H),7.10(dd,J 1 =5.6Hz,J 2 =8.6Hz,4...
Embodiment 2
[0037] Example 2: Preparation of N-1-benzyl-1-(4,4-bis(4-fluorophenyl))butylamine (Compound I-2):
[0038]
[0039] Add 4,4-bis(4-fluorophenyl)-1-butaneamine (compound II-2, 1.87g, 7.16mmol) and benzyl chloride (compound III-2, S0154, 0.45g, 3.58mmol) Into DMSO (16 mL) in a 50 mL single-necked reaction vial with magnetic stirring and stir at 80 °C for 7 h. The reactant was transferred to a 50mL separatory funnel, diluted with 20mL of ethyl acetate, and then diluted with 5mL of 5% Na 2 CO 3 Wash twice with aqueous solution, then wash twice with 10 mL of water, and finally wash once with saturated 10 mL of NaCl aqueous solution. Then the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain N-1-benzyl-1-(4,4-bis(4-fluorophenyl))butylamine (compound I-2 , 1.1g, 88% yield). 1 H-NMR (CDCl 3 ,400MHz):7.33~7.27(m,5H),7.10(dd,J 1 =5.5Hz,J 2 =8.6Hz,4H),6.92(t,J=8.6Hz,4H),3.82(s,2H),3.80(t,J=7.7Hz,1H),2.6...
Embodiment 3
[0040] Example 3: Preparation of N-1-(4,4-bis(4-fluorophenyl))butyl-1-(1H-2-indole)carboxamide (Compound I-3, S0161):
[0041]
[0042] (1H-2-indole) formic acid (compound III-3, 0.50g, 3.1mmol) and thionyl chloride (SOCl 2 , 5mL) into a 25mL single-port reaction flask with magnetic stirring, and stirred at reflux for 3 hours. Excess thionyl chloride was then removed under reduced pressure, followed by the addition of 5 mL of 4,4-bis(4-fluorophenyl)-1-butanean (compound II-3, 0.53 g, 2.06 mmol) in tetrahydrofuran and 4-(N,N-Dimethyl)pyridine (DMAP, 0.51g, 4.12mmol), and the reaction solution was stirred at room temperature for 12 hours. The reactant was transferred to a 50 mL separatory funnel, diluted with 20 mL of ethyl acetate, and then diluted with 20 mL of 5% Na 2 CO 3 Wash twice with aqueous solution, then wash twice with 10 mL of water, and finally wash once with saturated 10 mL of NaCl aqueous solution. Then the organic layer was dried over anhydrous sodium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com