Dry powder inhalant of interferon alpha
A dry powder inhaler and interferon-α technology, which is applied in antiviral agents, respiratory diseases, medical preparations of non-active ingredients, etc., can solve problems that do not involve the optimization of the subtype and content of the main drug interferon-α
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of the dry powder inhalation particle of interferon alpha
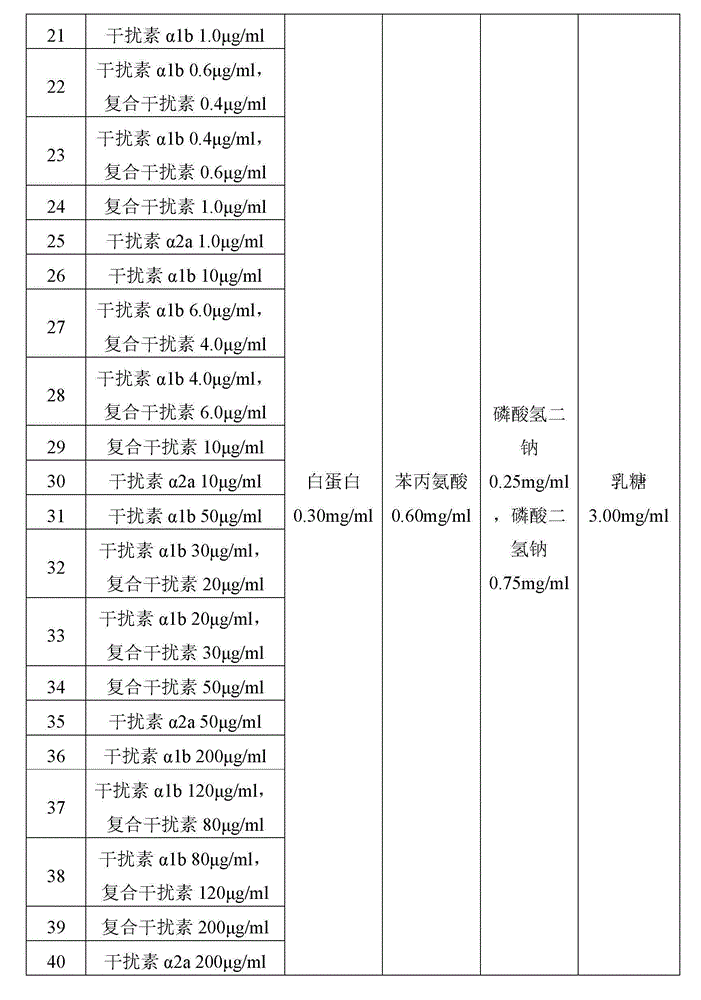
[0038] Prepare each solution for spray drying according to the formula in Table 1 below, and spray dry the solution with a Buchi B-290 spray dryer according to the corresponding spray drying conditions in Table 2 (continue to maintain the inlet temperature and flow rate of the spray drying gas after the spraying of all liquids is completed) 15 minutes) to prepare dry powder inhalation particles of interferon α.
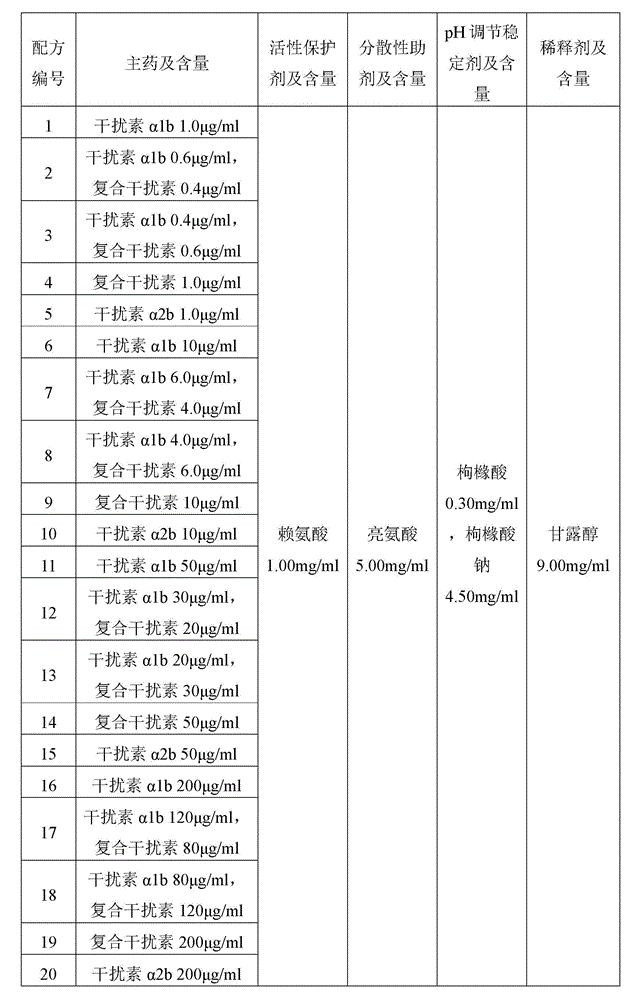
[0039] Table 1 Preparation of the solution formulation for spray drying of the dry powder inhaler granules of interferon α
[0040]
[0041]
[0042] Table 2 The spray drying conditions of the dry powder inhaler of interferon α
[0043]
Embodiment 2
[0044] Embodiment 2: the quality evaluation of the dry powder inhaler particle of interferon alpha
[0045] According to the provisions of the "Chinese Pharmacopoeia 2010 Edition (Part Three)" appendix "Interferon Biological Activity Assay Method", the activity of interferon α in the solution after the dissolution of the prepared interferon α dry powder inhalation particles of each formula (IU / ml); According to the provisions of the second method Lowry method of "Protein Content Determination Method" in the appendix of "Chinese Pharmacopoeia 2010 Edition (Part Three)", the interferon α in the solution of the prepared interferon α dry powder inhalation particles of each formula was determined after dissolution The concentration (mg / ml) was divided by the two to obtain the specific activity (IU / mg) of interferon α in the prepared interferon α dry powder inhalation particles of each formula. The specific activity of interferon α in each solution for spray drying was measured by ...
Embodiment 3
[0052] Embodiment 3: the dry powder inhaler of interferon alpha is prepared by the dry powder inhaler particle of interferon alpha
[0053] The dry powder inhalation particle sample of the interferon alpha of the formula 1-20 in each formula obtained by the method of Example 1 is not mixed with the large particle size carrier, and is directly packed with the amount of 20 mg per capsule as the interferon alpha. Dry powder inhalation is used for the research of embodiment 4-5; The dry powder inhalation particle sample of the interferon alpha of formula 21-40 mixes with commercially available large-size lactose carrier particle with the mass ratio of 1:3, with the dosage of 20mg per capsule. The amount was subpackaged and used as a dry powder inhaler of interferon α for the research of Examples 4-5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com