Chlorine-doped modified lithium ion battery lithium-rich cathode material and preparation method thereof
A lithium-rich cathode material, lithium-ion battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of rapid decline in specific capacity and poor rate performance, and achieve excellent cycle stability, excellent rate performance, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
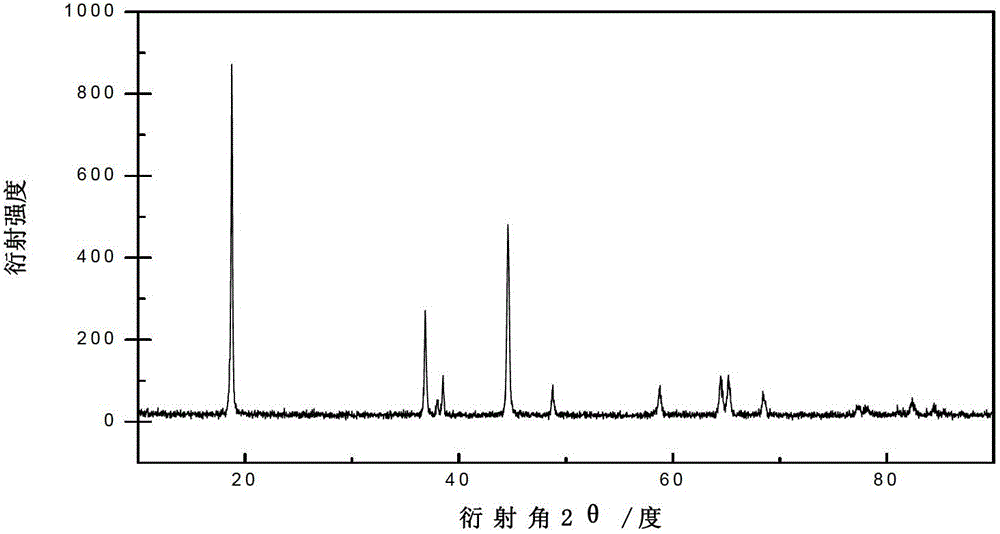
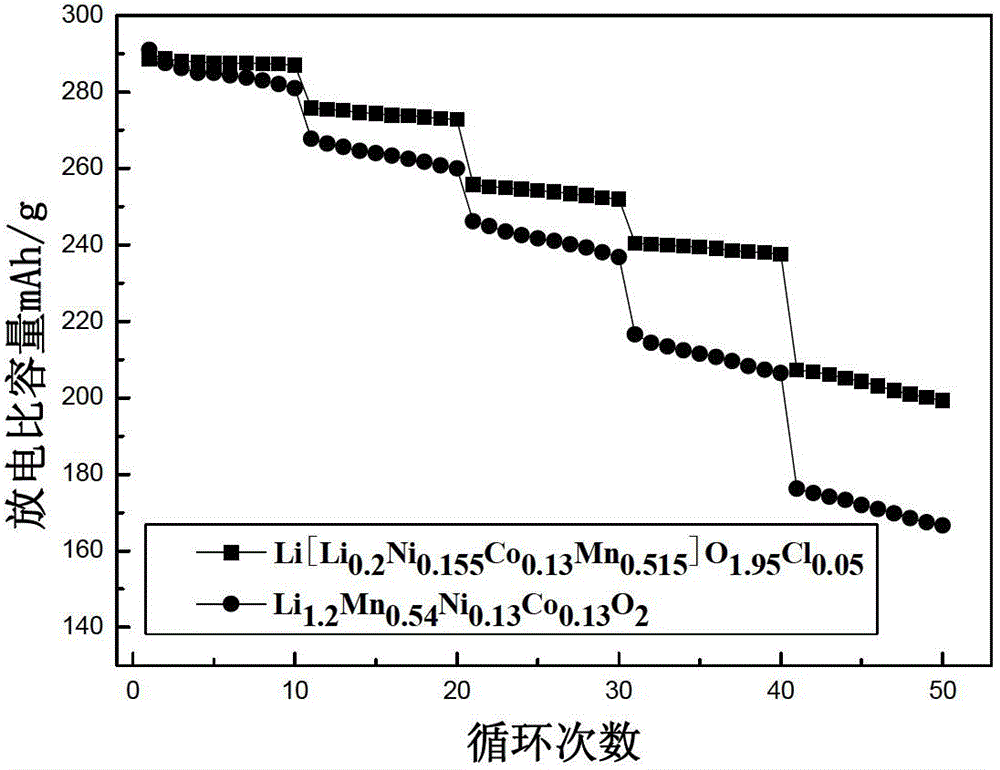
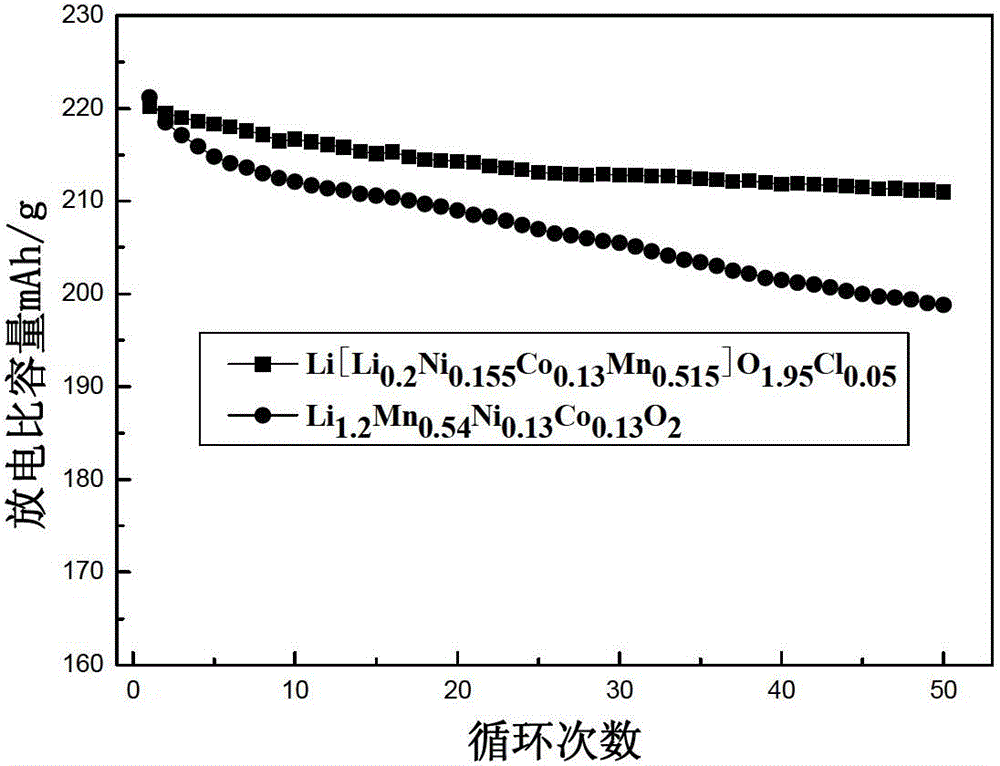
Image
Examples
Embodiment 1
[0024] Press LiNO 3 , Ni (NO 3 ) 2 , Mn (CH 3 COO) 2 , Co (NO 3 ) 2 , LiCl, and citric acid with a molar ratio of 1.21︰0.155︰0.515︰0.13︰0.05︰0.8 were weighed, ground in a mortar and mixed evenly, then added 5 mL of deionized water, and continued to grind until the mixture was uniform and fine mushy. Then bake the mixture at 120°C for 12 hours to obtain a precursor. The precursor is pre-calcined at 500°C for 6 hours, then cooled and ground, then calcined at 850°C for 20 hours and then cooled to obtain Li[Li 0.2 Ni 0.155 co 0.13 mn 0.515 ]O 1.95 Cl 0.05 Cathode material.
[0025] Press LiNO 3 , Ni (NO 3 ) 2 , Mn (CH 3 COO) 2 , Co (NO 3 ) 2 , The molar ratio of citric acid is 1.26:0.13:0.54:0.13:0.8 by weighing, and then the same steps as above can be used to synthesize Li 1.2 mn 0.54 Ni 0.13 co 0.13 o 2 .
Embodiment 2
[0027] Press LiNO 3 , Ni (NO 3 ) 2 , Mn (CH 3 COO) 2 , Co (NO 3 ) 2 , LiCl, and citric acid with a molar ratio of 1.23︰0.145︰0.525︰0.13︰0.03︰0.8 were weighed, ground in a mortar and mixed evenly, then added 5 mL of deionized water, and continued to grind until the mixture was uniform and fine mushy. Then bake the mixture at 120°C for 12 hours to obtain a precursor. The precursor is pre-calcined at 500°C for 6 hours, then cooled and ground, then calcined at 850°C for 20 hours and then cooled to obtain Li[Li 0.2 Ni 0.145 co 0.13 mn 0..525 ]O 1.97 Cl 0.03 Cathode material.
Embodiment 3
[0029] Press LiNO3 , Ni (NO 3 ) 2 , Mn (CH 3 COO) 2 , Co (CH 3 COO) 2 , LiCl, and citric acid with a molar ratio of 1.16︰0.18︰0.49︰0.13︰0.1︰0.8 were weighed, ground in a mortar and mixed evenly, then added 5 mL of deionized water, and continued to grind until the mixture was uniform and fine mushy. Then bake the mixture at 120°C for 12 hours to obtain a precursor. The precursor is pre-calcined at 550°C for 5 hours, cooled and ground, then calcined at 850°C for 20 hours and then cooled to obtain Li[Li 0.2 Ni 0.18 co 0.13 mn 0.49 ]O 1.9 Cl 0.1 Cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com