Synthesis method of cubic boron nitride and preparation method of cubic boron nitride sintered body
A technology of cubic boron nitride, synthesis method, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
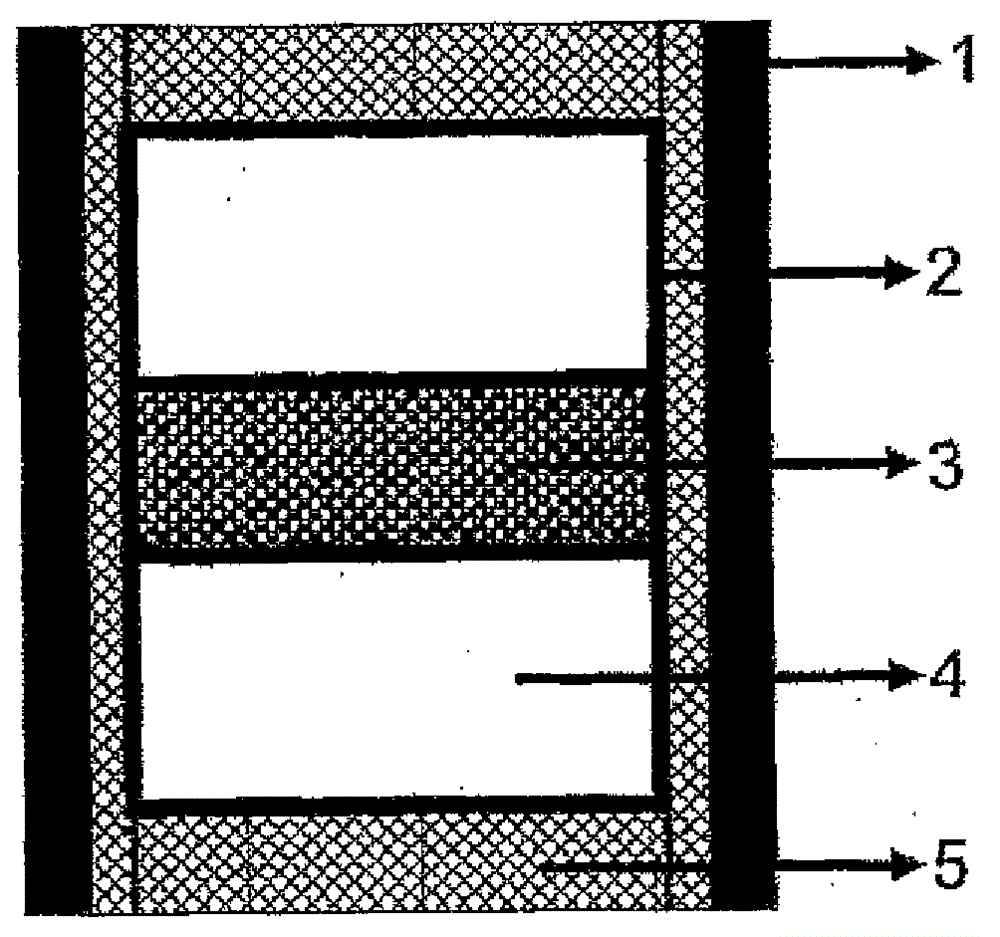
[0064] Such as figure 1 As shown, the alloy powder of the composition composed of Co60-Mo20-V16-Al4 mass % is made in advance as a metal catalyst, and it is filled to an outer diameter of 10 mm, an inner diameter of 7 mm and a height of 7.6 mm (including 10 mass % of Zirconia powder) molded body 5 in the sample container so as to form a metal catalyst (alloy powder) layer 3 with a thickness of about 1.6 mm sandwiched between two hBN molded plates 4 with a diameter of about 7 mm and a thickness of 3 mm.
[0065]The sample is surrounded by a graphite tubular heater 1 with an outer diameter of 12 mm, an inner diameter of 10 mm, and a height of 17.6 mm. Mo foil 2 is arranged on the inner surface, and a belt-type ultra-high pressure device is used (the front end diameter of the anvil is 21 mm, and the inner diameter of the cylinder is 25 mm). Pressurize, then put electricity into the heater to heat, and keep it for a certain period of time, then drop the temperature rapidly, and th...
Embodiment 2
[0071] Using an alloy powder of a composition composed of Co40.87-Mo17.39-Cr20.87-V18.26-Al2.61% by mass as a metal catalyst, in the same manner as in Example 1, at a pressure of 4.6GPa and 1330°C As a result of cBN synthesis by reacting for 60 minutes, microparticle cBN with a cBN conversion rate of 90% or more and an average particle diameter of 20 μm was synthesized.
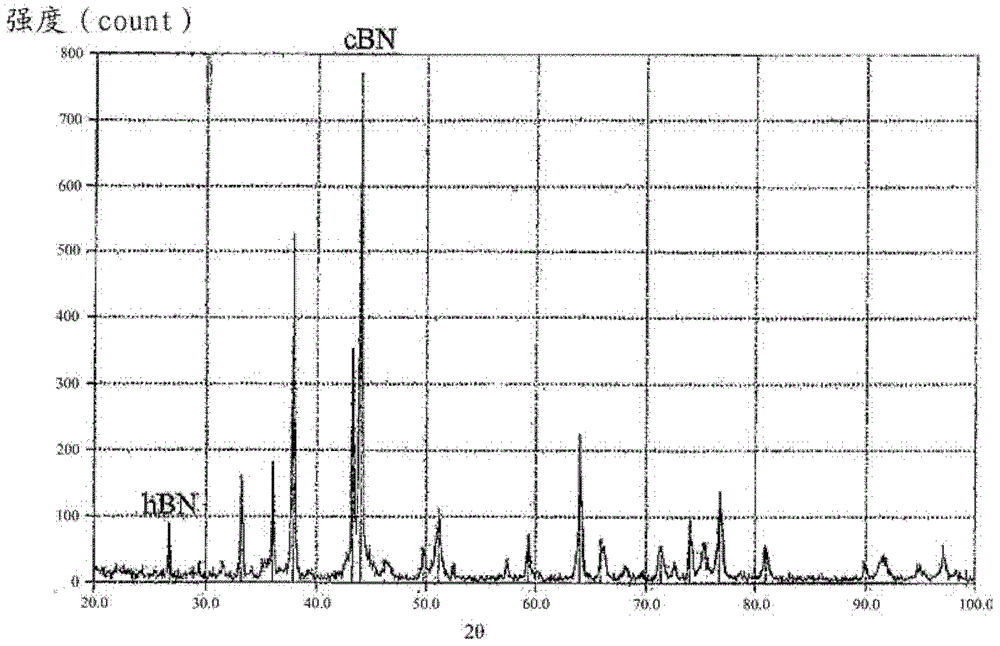
[0072] image 3 The X-ray diffraction curve obtained for the microparticle cBN obtained in Example 2 is shown in .
[0073] and, Figure 4 A photograph of the structure of the microparticle cBN obtained in Example 2 observed by a scanning electron microscope is shown in .
Embodiment 3
[0075] As a metal catalyst, cBN was synthesized by reacting for 30 minutes at a pressure of 4.6 GPa and 1320°C in the same manner as in Example 1, using an alloy powder having a composition composed of Co47-Mo20-Cr24-V6-Al3 mass %. Microparticle cBN with a cBN conversion rate of 80% or more and an average particle size of 20 μm was obtained. In addition, a very small amount of cBN with an average particle size of about 40 μm was also synthesized at the same time. As described above, if the added amount of V is reduced, the distribution of V becomes locally uneven, and cBN particles grow significantly to 30 μm or more in the portion where the concentration of V is low.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com