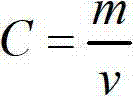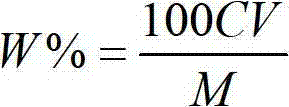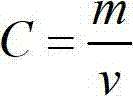Method for analyzing copper in copper-containing ferric trichloride with low electronic grade
A copper ferric chloride and analysis method technology, applied in the field of chemical analysis, can solve the problems of interference measurement, high minimum copper concentration, color affecting colorimetry, etc., to achieve the effect of eliminating interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] The method for analyzing copper in low electronic grade copper-containing ferric chloride comprises the steps:
[0017] one. instrument
[0018] UV-756CM spectrophotometer, 10ml cuvette, 50ml colorimetric tube, PH meter or precision PH test paper, 100ml volumetric flask, filter paper, funnel, rubber ball, 25ml pot-bellied straw
[0019] two. Reagent
[0020] Copper standard stock solution: Weigh 1.0000g of spectrally pure metal copper, dissolve it in 20ml of 1:1 nitric acid, after cooling, dilute it in a 1000ml volumetric flask with pure water, shake well, 1.00ml of this solution contains 1.00mg of Cu. Take the copper standard stock solution and dilute it into the required standard solution, such as 10.0 μg Cu in 1.00 ml or 50.0 μg Cu in 1.00 ml.
[0021] Saturated sodium fluoride solution: Weigh 4.06g of sodium fluoride, dissolve it in a 100ml volumetric flask.
[0022] 40% triammonium citrate solution: weigh 40g of triammonium citrate, dissolve in pure water, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com