Carbonyl propyl sulfuryl anthracene pyridone sulfonic acid compound, preparation method and application thereof
A compound, sulfonic acid technology, applied in the direction of benzo-azabenzoxanthrone dyes, applications, household appliances, etc., can solve the problem that dyes have not yet met the color tone, vividness, light resistance, water resistance, ozone resistance and dissolution. problems such as stability and solution stability, insufficient dye solubility stability, insufficient long-term stability, etc., to achieve the effects of high long-term stability, image light fastness, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
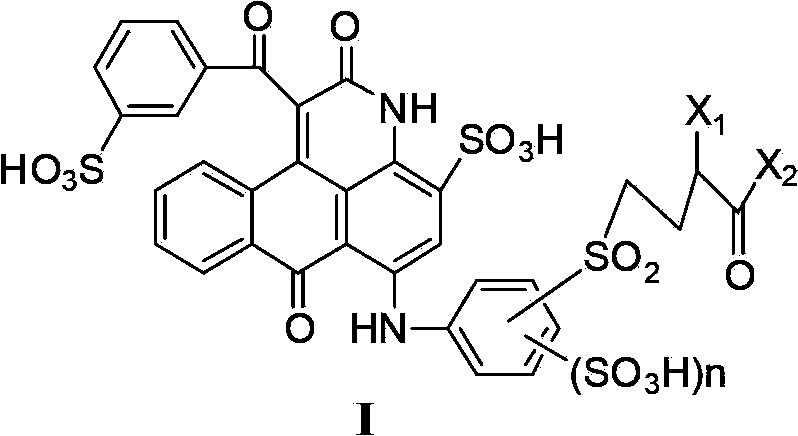
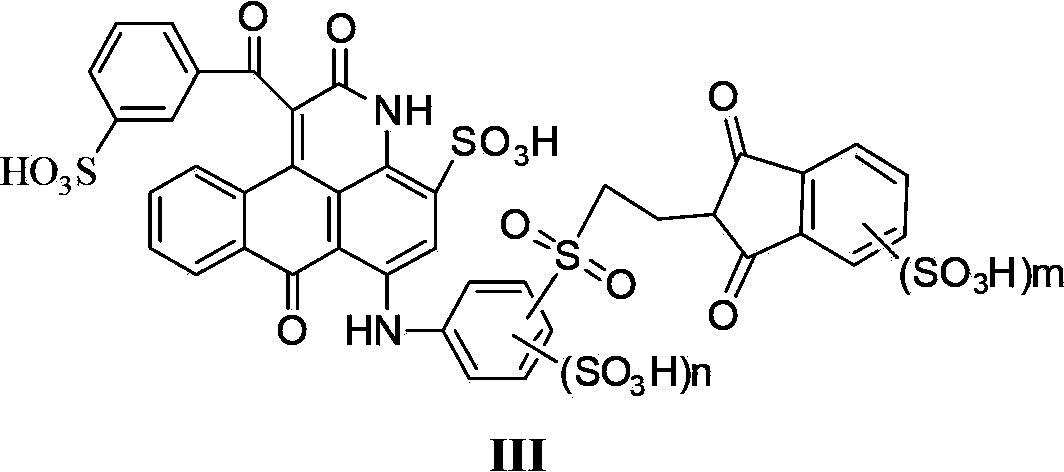
[0145] The preparation of the ink composition of the present invention is: the dye compound represented by the general formula (I) or (III) or its salt or their mixture is dissolved in water or the above-mentioned aqueous solvent (water containing a water-soluble organic solvent) Or in a water-miscible organic solvent, it can be prepared by dissolving it together with the above-mentioned ink control agent and the like as needed.
[0146] In the above production method, there is no particular limitation on the order in which the components are dissolved. The dye may be dissolved in water or the above-mentioned aqueous solvent (water containing a water-soluble organic solvent), and the ink control agent may be added to dissolve the dye, or an aqueous solvent or an ink preparation agent may be added after dissolving the dye in water. The order may be different from this. Furthermore, an ink composition can be produced by adding an aqueous solvent and an ink preparation agent to ...
Embodiment 1
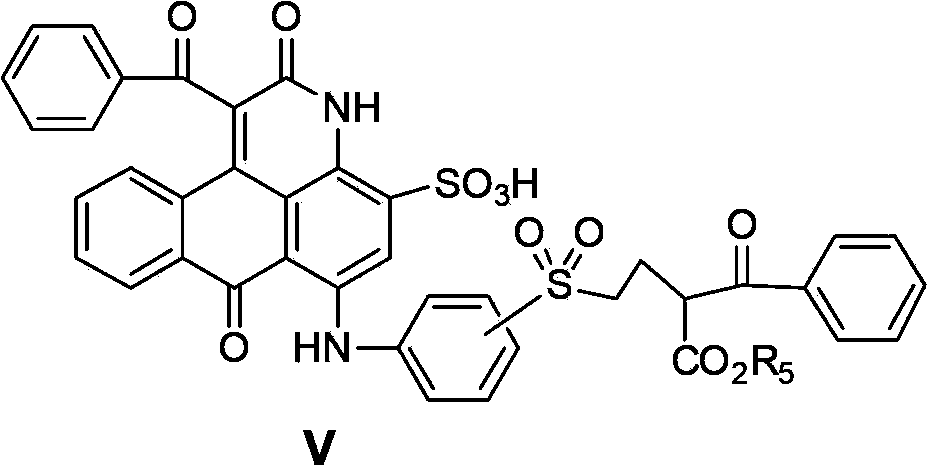
[0158] (1) In 350 parts of o-dichlorobenzene, add 100 parts of dimethyl sulfoxide, and add 160 parts of (C.I. Reactive Blue 19) derivative (sodium salt, formula V'-RB19), 10 parts in sequence while stirring Parts of sodium carbonate, 250 parts of ethyl benzoyl acetate and heating. Carried out the reaction at 175 to 180°C for 4 hours, during which the by-product ethanol and water generated in the reaction were discharged from the reaction system by azeotropic distillation, and the color gradually changed from blue to purple, and the liquid chromatograph detected that the reaction was complete (about 4 Hour). Cooling, at M -H] -1 ). The most abundant and accurate molecular mass of the intermediate dye product V-RB19 (in the form of free sulfonic acid) M for 804.1.
[0159]
[0160] (2) In 450.0 parts of 95.0% sulfuric acid, under cooling and stirring, add 380.0 parts of 50% oleum to prepare 830 parts of 10% oleum. After cooling, at a temperature not higher than 40°C, add...
Embodiment 2
[0171] The sodium salt of intermediate V-RB19 was prepared in the same manner as in step 1 of Example 1. Then 10% SO in the 2nd step sulfonation 3 h 2 SO 4 Replaced with 12% SO 3 h 2 SO 4 , Reaction temperature is brought up to 85-90 ℃, by the same method of embodiment 1 step 2, desalination obtains 2600 parts of solutions containing 185 parts of mixed dyes (M2, sodium salt form), and the maximum absorption wavelength in water is 533nm.
[0172] Adopt reverse-phase ion-pair liquid chromatography, according to the same separation device and method as in Example 1, the dye compound in the above-mentioned dye mixture M2 is separated, and what is washed out at first is the Dm6 pure compound of six charges; Then The five-charged Dm2 pure compound and Dm4 pure compound are washed out in sequence; then the four-charged dye Dm8 pure compound and Dm10 pure compound are sequentially washed out.
[0173] In the high-performance liquid chromatography HPLC analysis, according to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com