Wound dressing having bacteriostasis and hygroscopicity
A wound dressing and modified technology, which is applied in the direction of adhesive dressing, non-adhesive dressing, dressing, etc., and can solve the problem of not having antibacterial function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Material: chitosan fiber, fineness is 2.0dtex, length is 50 mm, treated with 1% by weight of surfactant and its hygroscopicity contains 8.298 grams of sodium chloride and 0.368 grams of sodium chloride per liter in fiber state Calcium chloride dihydrate solution (solution A) has an absorption rate of 110%; modified cellulose fiber, fineness is 1.4dtex, viscose fiber with a length of 38 mm, treated with carboxymethyl and its hygroscopicity is in the fiber state The absorption rate for a solution (A solution) containing 8.298 grams of sodium chloride and 0.368 grams of calcium chloride dihydrate per liter was 2200%.
[0040] 100 grams of chitosan fibers and 1900 grams of carboxymethylcellulose fibers were uniformly mixed by hand for 5 minutes and then fed to a single cylinder card (Cuarnicard type), where the fibers were further mixed in the card hopper. The fiber web formed after carding is a homogeneous mixture of the fibers. Afterwards, this fiber web is made into nee...
Embodiment 2
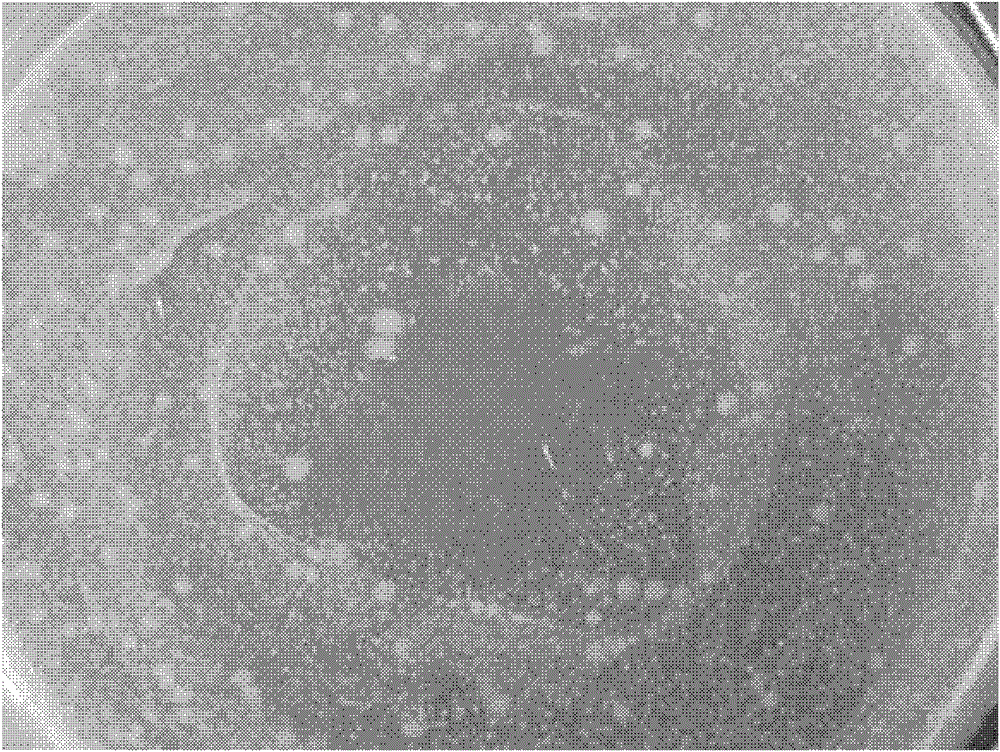
[0044] In order to observe the antibacterial properties of the wound dressing of Example 1, evenly coat 0.25ml of 10 6 -10 7 cfu / g of Staphylococcus aureus. The wound dressing according to Example 1 was then cut into 2 x 2 cm and placed in Petri dishes. Continuously culture at a constant temperature of 37°C and observe the growth of bacteria on each plate. figure 1 Bacterial growth / inhibition on the bottom of the wound dressing after 1 day in a Staphylococcus aureus Petri dish is shown for the wound dressing of Example 1.
[0045] From figure 1 According to visual observation, there is little bacterial growth at the bottom of the wound dressing, which shows the good antibacterial performance of the wound dressing of the present invention.
Embodiment 3
[0047] Material: Acylated chitosan fiber, fineness 2.2dtex, length 50 mm, after acylation treatment and its hygroscopicity, in the fiber state, it contains 8.298 grams of sodium chloride and 0.368 grams of calcium chloride dihydrate per liter The absorption rate of the solution (A solution) is 1500%; modified cellulose fiber, the fineness is 1.7dtex, the length is 50 mm, treated with carboxymethyl and its hygroscopicity can contain 8.298 grams of chlorine per liter in fiber state. The absorption rate of the solution (A solution) of sodium chloride and 0.368 g of calcium chloride dihydrate was 1500%.
[0048] Preparation of acylated chitosan:
[0049] Take by weighing the chitosan fiber of 400g, soak with dehydrated alcohol after 30min, dehydration, then chitosan fiber is placed in succinic anhydride-ethanol (894g succinic anhydride, be dissolved in the dehydrated alcohol of 8940ml) solution, 70 ℃ water bath heating for 40min. The fibers are then taken out, dehydrated, and wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com