Urea transporter inhibitors, and preparation method and application thereof
A compound and straight-chain technology, applied in the field of urea channel protein inhibitors, can solve the problem of no increase in urine osmotic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
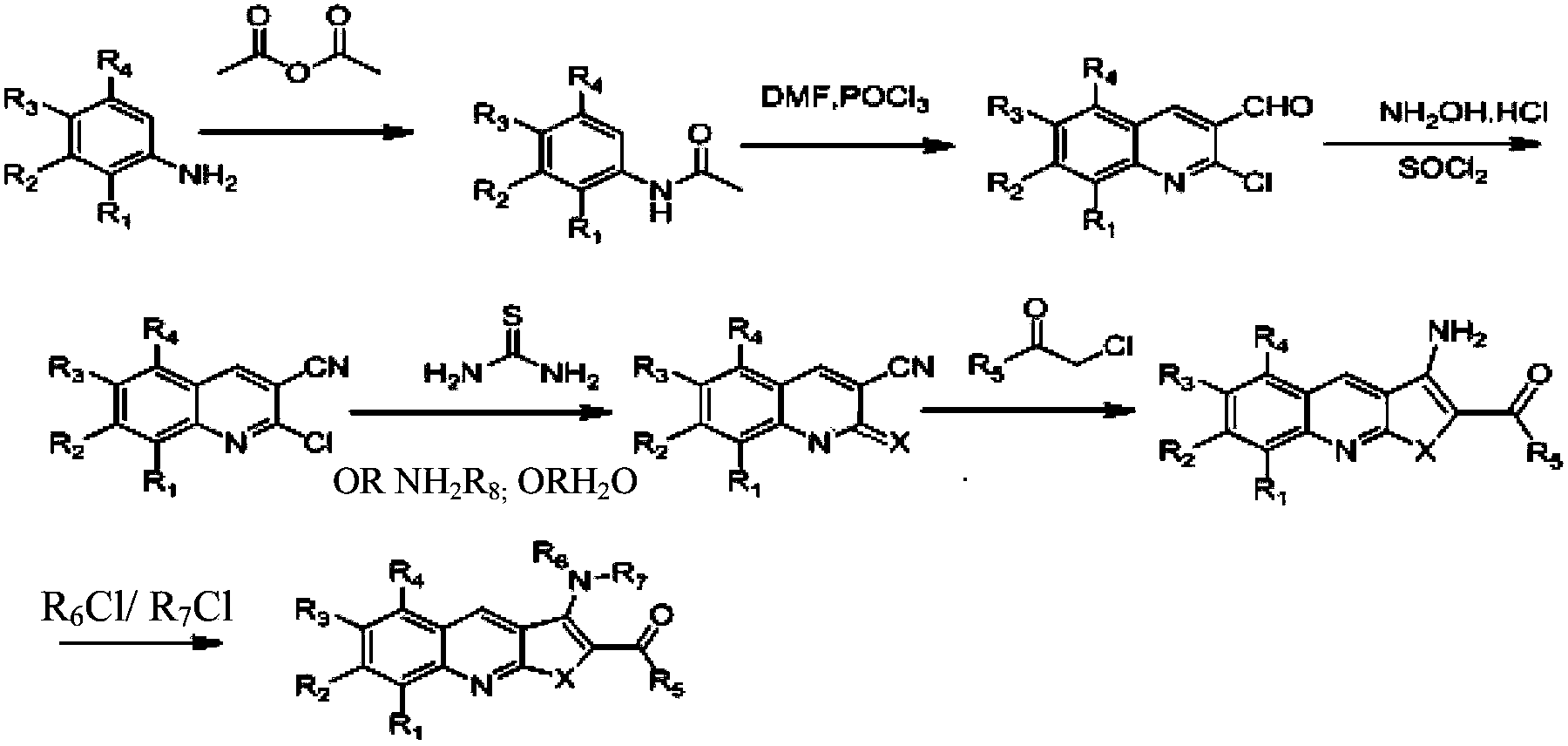
Embodiment 1
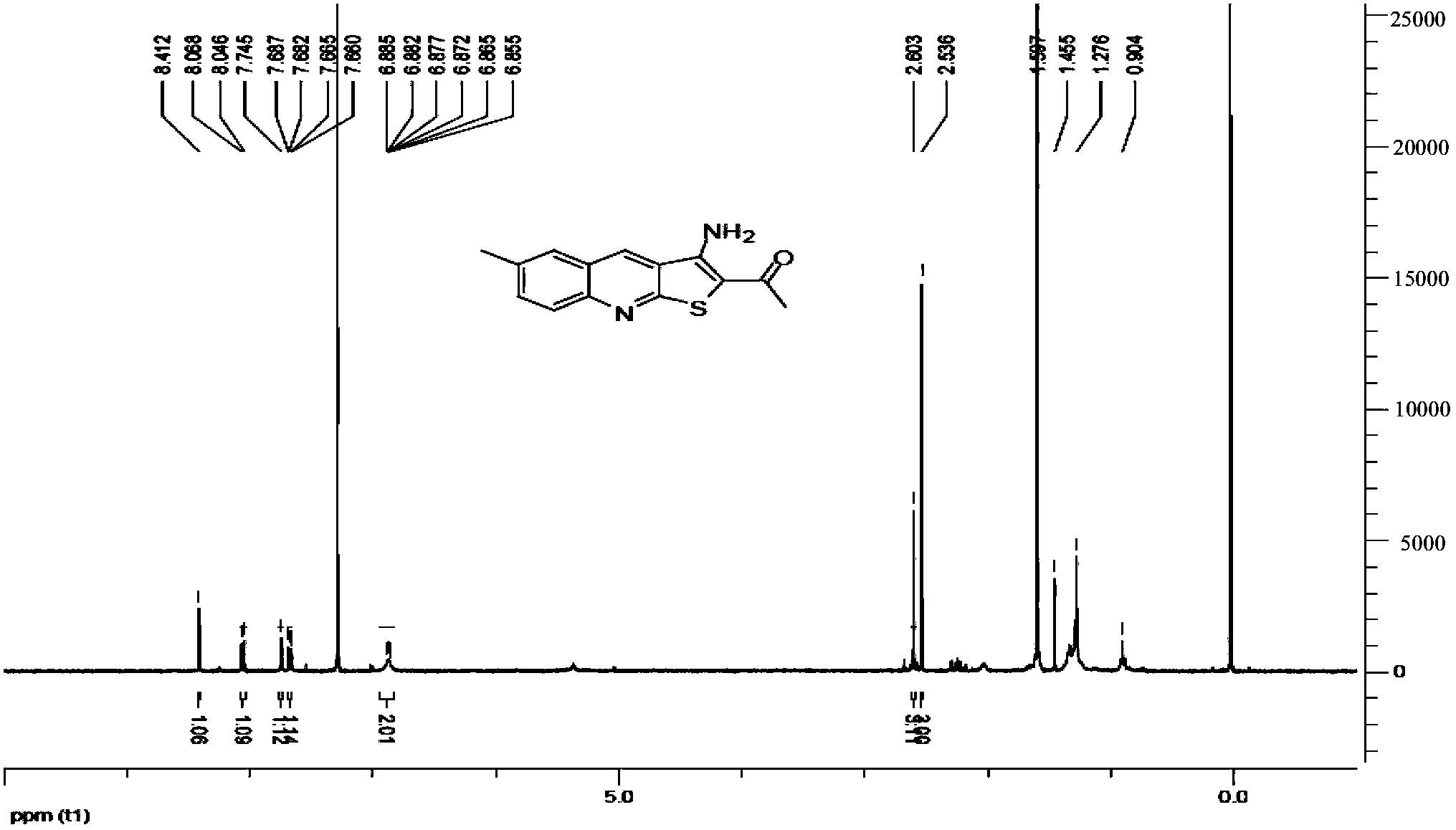
[0045] Example 1, Preparation of 1-[2-(3-amino-6-methylthieno[2,3-b]quinolyl)]ethanone (Youte, A-04)
[0046]
[0047] 1) Add 130ml of acetic anhydride to a three-neck flask, dissolve compound 1 (100g, 0.93mol) with 200ml of DCM (dichloromethane), add it dropwise to the acetic anhydride, and control the temperature at 30-40°C. After the dropwise addition, the temperature was kept for half an hour, and the reaction was monitored by TLC. Heat 600ml of water to 50°C, pour the reaction solution into hot water, stir for half an hour, and concentrate to DCM. The solid was filtered off with suction, and the solid was washed twice with 200 ml of water each time. Compound 2 (125 g) was obtained by drying, with a yield of 90%.
[0048] 2) Add 110ml of DMF (dimethylformamide) into the three-necked flask, cool down to 0°C, and dropwise add 360ml of POCl 3 , temperature control 0-10 degrees, there is solid precipitation in the middle, unable to stir, continue to add dropwise, the sys...
Embodiment 2
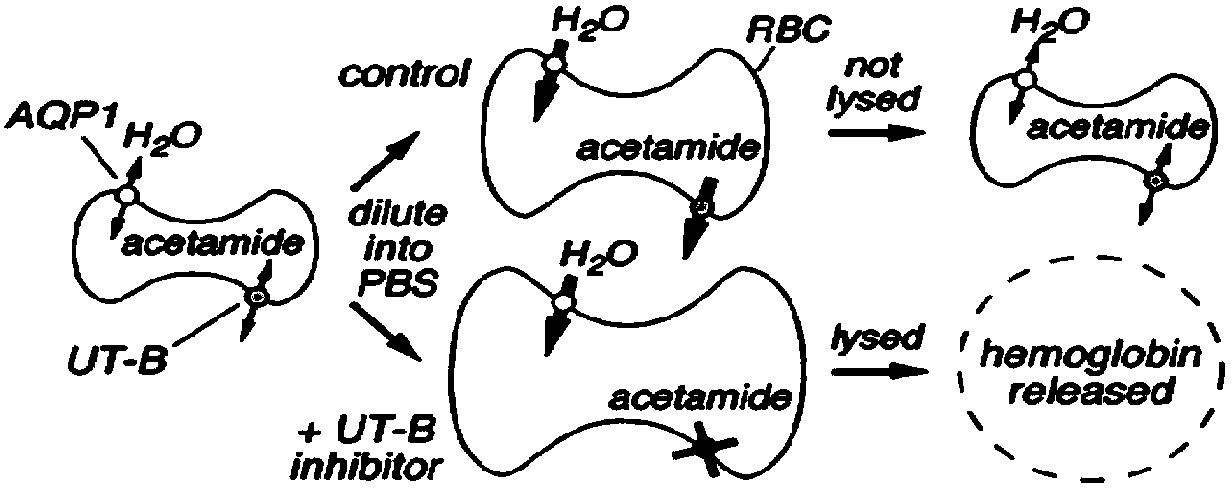
[0054] Example 2, Screening and Pharmacodynamic Evaluation of UT-B Inhibitors
[0055] 1. Screening test method
[0056] 1) Take blood, put it in a 15ml graduated centrifuge tube (suspended in PBS containing sodium heparin), centrifuge at 3000r / min for 10min, discard the supernatant;
[0057] 2) Add the same amount of PBS as the blood, centrifuge at 3000r / min for 10min, discard the supernatant;
[0058] 3) Dilute the red blood cells with hypertonic PBS containing 1.25M acetamide to a cell suspension with a specific volume of about 2%;
[0059] 4) The erythrocyte suspension was incubated at room temperature for 2 hours to balance the concentration of acetamide inside and outside the cells, and mixed with a pipette at regular intervals;
[0060] 5) Take 99 μl of the above-mentioned erythrocyte suspension and place it in each well of a 96-well round-bottom microplate, then add 1 μl of the compound to be tested (such as Utyl), mix well, and incubate at room temperature for 6 min (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com