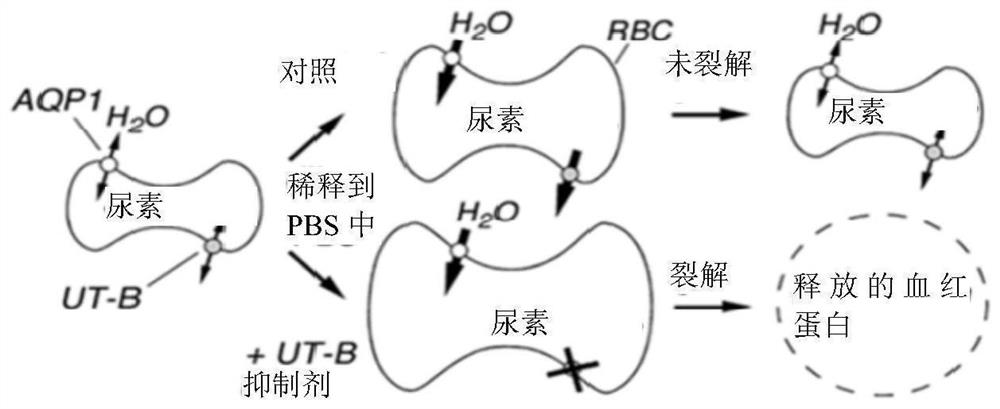
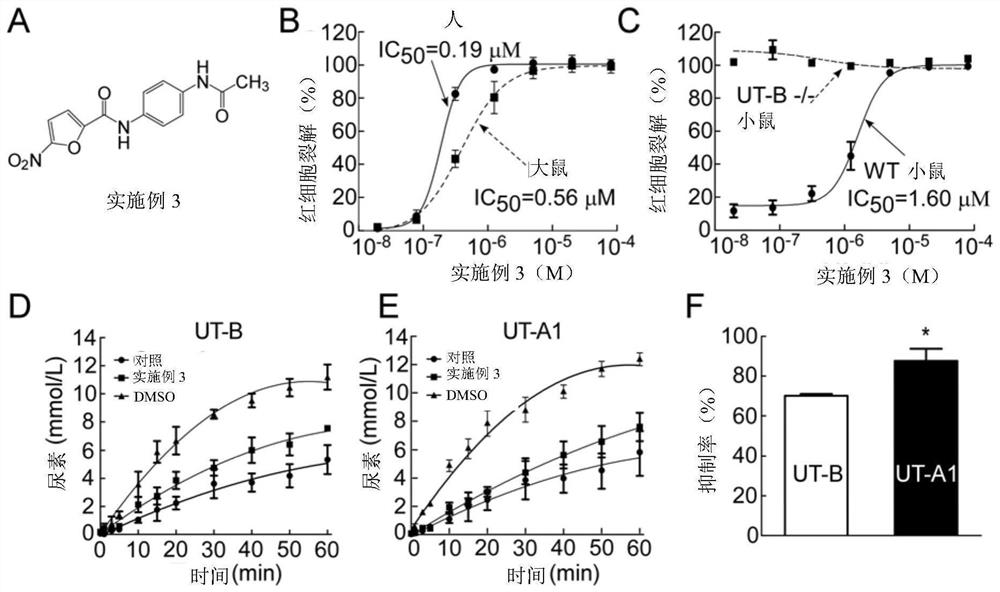
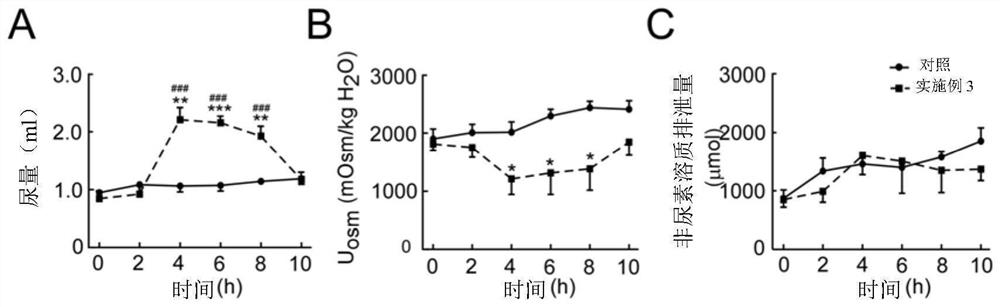
Diarylamide compound and application thereof
A compound, formamide technology, applied in the field of diuretics, can solve the problem of no increase in urine osmotic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] Example 1 N-(4-acetylaminophenyl)-4-nitrobenzamide
[0220]
[0221] Suspend p-nitrobenzoic acid (3-1a, 167mg, 1mmol) in dichloromethane (5mL), add 2 drops of DMF, and cool to 0°C in an ice-water bath. Oxalyl chloride (190mg, 0.13mL, 1.5mmol) was slowly added dropwise, and the mixture was stirred at room temperature for 2 hours. The reaction solution was concentrated to remove the solvent and excess oxalyl chloride to obtain p-nitrobenzoyl chloride (3-2a), which was added to tetrahydrofuran (1 mL) for later use without further purification. Paracetaminoaniline (3-3a, 150 mg, 1 mmol) was dissolved in tetrahydrofuran (5 mL), triethylamine (152 mg, 1.5 mmol) was added, and the tetrahydrofuran solution of the above acid chloride 3-2a was added dropwise under ice bath. After dropping, react at room temperature to TLC (CH 2 Cl 2 :MeOH=15:1) showed that the reaction was complete. Add 30 mL of water to the reaction system, continue to stir for 10 minutes and then filter ...
Embodiment 2
[0223] Example 2 N-(4-acetylphenyl)-5-nitrofuran-2-carboxamide
[0224]
[0225] Synthetic method is the same as embodiment 1. Yellow solid, yield 81%, melting point 233-234°C.
[0226] 1 H NMR (400MHz, DMSO-d 6 )δ10.91(s,1H),7.99(d,J=8.0Hz,2H),7.90(d,J=8.0Hz,2H),7.82(d,J=2.4Hz,1H),7.69(d, J=2.4Hz,1H),2.55(s,3H); 13 C NMR (101MHz, DMSO-d 6 )δ197.13, 155.33, 152.36, 147.92, 142.68, 133.14, 129.84, 120.31, 117.57, 113.90, 26.98.
Embodiment 3
[0227] Example 3 N-(4-acetylaminophenyl)-5-nitrofuran-2-carboxamide
[0228]
[0229] Synthetic method is the same as embodiment 1. Orange solid, yield 80%, melting point 233-234°C.
[0230] 1 H NMR (400MHz, DMSO-d 6 )δ10.59(s,1H),9.97(s,1H),7.81(d,J=4.0Hz,1H),7.62-7.66(m,3H),7.57(d,J=8.8Hz,2H), 2.04(s,3H); 13 C NMR (101MHz, DMSO-d 6 )δ168.61, 154.79, 152.20, 148.56, 136.49, 133.32, 121.66, 119.71, 116.77, 113.97, 24.40. HRMS m / z: calcd for C 16 h 13 N 3 o 4 ,([M+H]+):285.08698,Found:285.08696.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com