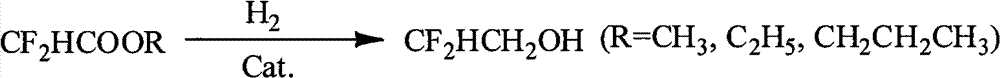Method for preparing difluoroethanol
A technology of difluoroethanol and difluoroacetate, applied in the preparation of organic compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of low reaction yield and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 3.2g of the catalyst prepared above, 40g of methyl difluoroacetate and 40g of absolute ethanol into a stainless steel autoclave, close the autoclave, and feed 1MPa H 2 Replace the air in the reactor, then feed hydrogen gas to a pressure of 5 MPa, heat to 250°C with stirring, and react for 10 hours. After the reaction is over, cool down, and when the temperature drops to 25°C, release the pressure, open the reaction kettle, remove the reaction liquid, filter the reaction liquid, separate the catalyst from the reaction liquid, and rectify the resulting filtrate at atmospheric pressure to collect fractions at 97-99°C , to obtain 26.9 g of difluoroethanol with a yield of 90.3% and a purity of 99%.
Embodiment 2
[0017] Add 3.2g of the catalyst prepared above, 20g of ethyl difluoroacetate and 60g of absolute ethanol into a stainless steel autoclave, close the autoclave, and feed 1MPa H 2 The air in the reactor was replaced, and then the pressure of hydrogen was 10MPa, heated to 180°C under stirring, and reacted for 8h. After the reaction, cool down, and when the temperature drops to 25°C, release the pressure, open the reaction kettle, take out the reaction liquid, filter, separate the catalyst from the reaction liquid, rectify the filtrate at atmospheric pressure, collect the fraction at 97-99°C, and obtain the product Difluoroethanol 12.1g, yield 91.6%, purity 99%.
Embodiment 3
[0019] Add 3.2g of the catalyst prepared above, 30g of propyl difluoroacetate and 50g of absolute ethanol into a stainless steel autoclave, close the autoclave, and feed 1MPa H 2 The air in the reactor was replaced, and then the pressure of hydrogen gas was introduced to 8 MPa, and the temperature was raised to 200° C. under stirring, and the reaction was carried out for 6 hours. Cool and lower the temperature. After the temperature drops to 25°C, release the pressure, open the reaction kettle, take out the reaction solution, filter, separate the catalyst from the reaction solution, rectify the filtrate at atmospheric pressure, collect fractions at 97-99°C, and obtain 16.22 g of difluoroethanol , yield 91.0%, purity 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com