Compositions and methods for increasing serum half-life of Fc fusion proteins
A fusion protein, half-life technology, applied in chemical instruments and methods, drug combinations, fusion polypeptides, etc., can solve problems such as half-life changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Embodiment 1: Expression of sugar variant TNFR2-Fc fusion protein
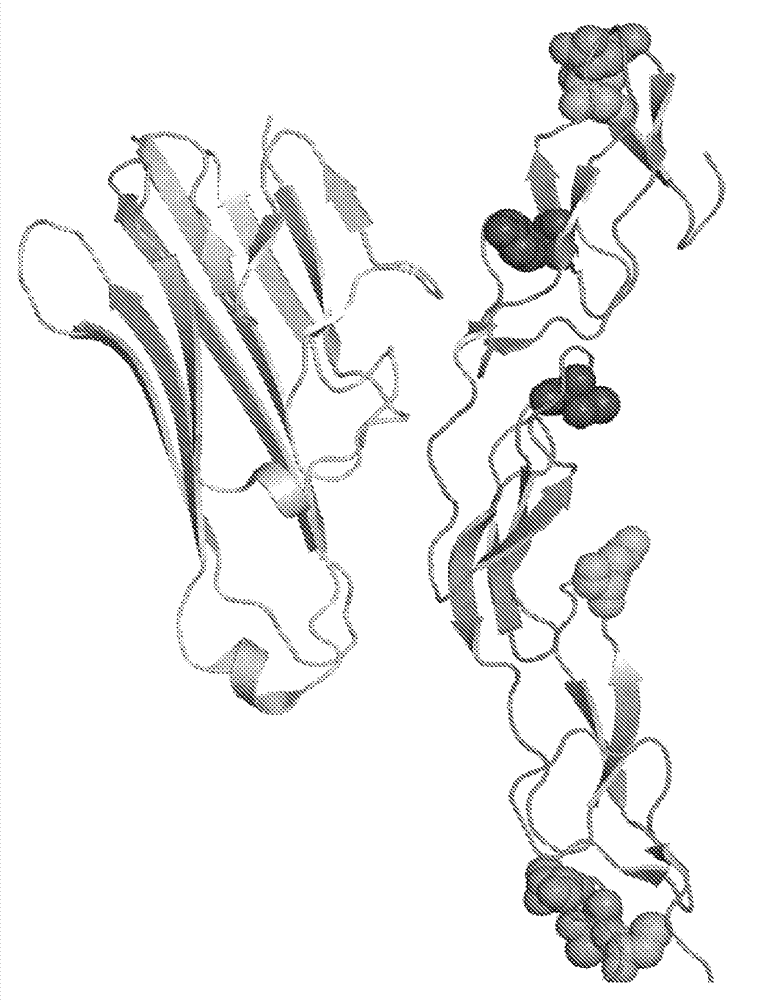
[0164] Applicants constructed vectors for expressing various TNFR2-Fc fusion proteins, each of which contains additional N-linked glycosylation sites. A control TNFR2-Fc fusion protein (ie, the basic or "original" unmodified form of TNFR2-Fc) had the extracellular domain of human TNFR2 fused to the human IgGl Fc domain without an inserted linker. exist figure 1 The sequence of the original TNFR2-Fc fusion protein (SEQ ID NO:5) is shown in Figure 5 The encoding nucleic acid (including the leader sequence) is shown in (SEQ ID NO:8). figure 1 The fusion protein shown is the more fully labeled TNFR2-h(1) Fc. like Figure 9 As shown, the variant designated TNFR2-h(2)Fc has an alternative linker / border sequence (SEQ ID NO: 16) and is derived from Figure 10 The nucleotide sequence shown (SEQ ID NO: 17) encodes. The protein construct was cloned into the pAID4 vector for expression in mammalian cells (...
Embodiment 2
[0173] Embodiment 2: Design of sugar variant TNFR2-Fc fusion protein
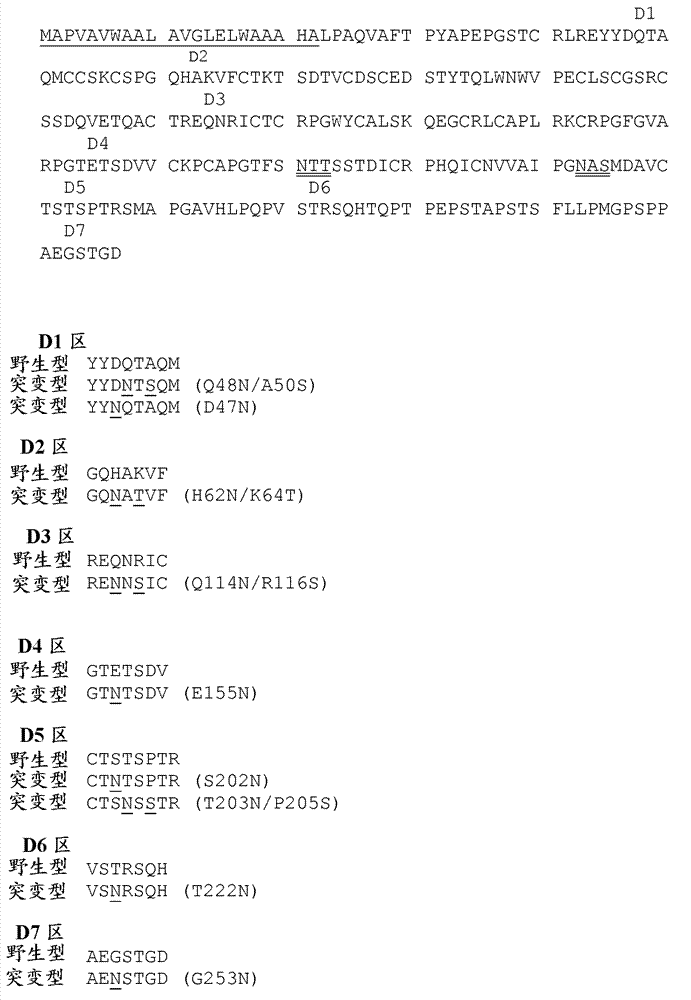
[0174] The location to introduce additional N-linked glycosylation sites was chosen by examining the co-crystal structure of the TNFR2 ectodomain with its ligand TNF (crystal coordinates are publicly available), and by applying the principles described herein. Select Changes in the Figure 7 As shown in , changes in this position are predicted to preserve the TNF antagonist activity of the protein while conferring an extended half-life. Note that the amino acid numbering is based on the native unprocessed TNFR2 amino acid sequence, eg Figure 7 shown. Altered TNFR2-Fc fusion proteins were designed with an additional N-linked carbohydrate moiety at one of the following positions: 47, 48, 62, 114, 155, 202, 203, 222, and 253. Note that native TNFR2-Fc has N-linked sugar moieties at positions 171 and 193, which means that the ratio of N-linked sugar moieties to amino acids in the heterologous domain of th...
Embodiment 3
[0175] Embodiment 3: Test TNFR2-Fc sugar variant protein
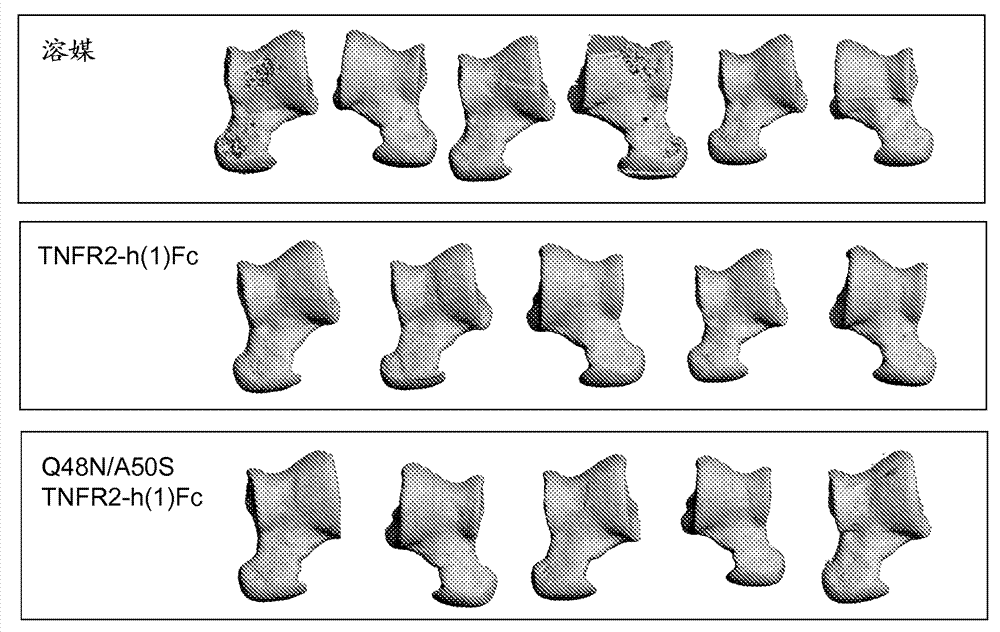
[0176] Glycovariant variants of TNFR2-h(1) Fc were tested in a series of assays to evaluate the functionality of the modified molecules and the effect of the modifications on the pharmacokinetic properties of the molecules.
[0177] For the evaluation of ligand binding in cell-free biochemical assays, a Biacore® 3000 biosensor was used. Briefly, TNFR2-h(1) Fc variants were added to a flow cell and then exposed to TNF. By evaluating the kinetic parameters (k a and k d ) to calculate the dissociation constant (K D ).
[0178] To assess ligand inhibition, a cell-based assay for TNF signaling was used essentially as described by Khabar et al. Immunol. Lett. 46 (1995): 107-110. Briefly, WEHI cells (ATCC) were cultured in the presence of TNF-α (R&D Systems, Minneapolis, Minn.) and actinomycin D. TNF-α induced apoptosis of these cells, and in A 490nm Check the cleavage rate. The presence of a TNF-[alpha] antagonist ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com