Method for effectively expressing recombinant human coagulation factor VIII
A high-efficiency expression and human blood coagulation technology, applied in the field of bioengineering, can solve the problems of low expression of the eight factors, complex cell line construction, and expression levels that cannot reach industrialization, and achieve low construction difficulty, improved accumulation and stability, and easy Magnified effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Experimental method
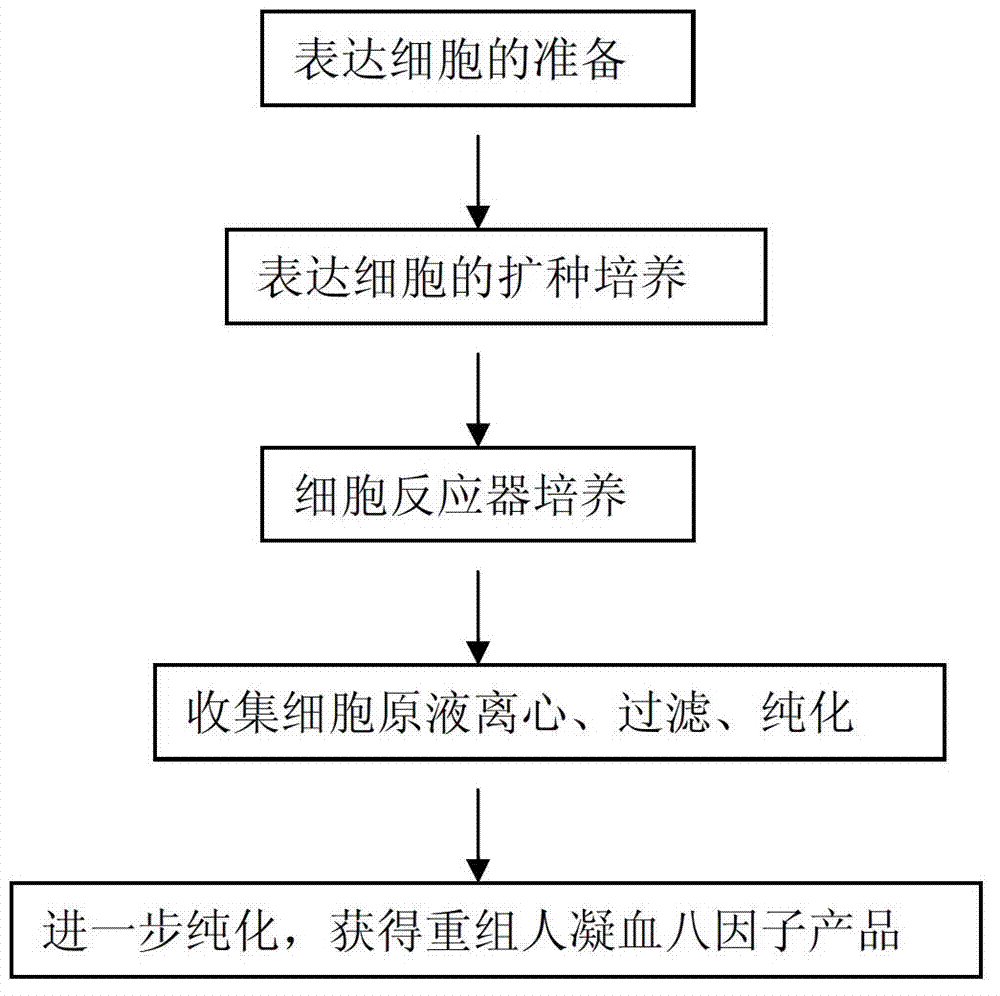
[0034] 1) Acclimate into serum-free cultured eight-factor expressing cells (construction of eight-factor expressing cells reference "Expression of blood coagulation factor FVIII in mammalian cells", Wang Qi et al., Pharmaceutical Biotechnology, 2002, 9 (5) : 251~255; the serum-free acclimatization method of cell lines can refer to the cell culture manual, such as the cell culture operation part in the appendix of the product manual of Invitrogen Company), with 9×10 5 cells / ml to inoculate the Erlenmeyer shaker flask, the culture temperature is 32°C, and the shaker flask speed is 60 rpm; after about 3 days of serum-free culture, the cell density reaches 2×10 6 cells / ml, passaged at a ratio of 1:2, cultured in shake flasks, cultured at 38°C, rotated at 60 rpm, until the cell density reached 1-2×10 6 cells / ml. The serum-free cell culture medium is commercialized SFMII302 medium produced by SIGMA Company.
[0035]2) Change the culture medium cont...
Embodiment 2
[0058] 1) Construction of cell lines expressing human coagulation factor VIII
[0059] The preparation method of the cell line expressing human coagulation factor VIII is the same as that in Example 1.
[0060] 2) Expansion culture of cells expressing eight factors
[0061] Take 1×10 5 Inoculate the Erlenmeyer shake flask with cells / ml, culture at 37℃ without serum for about 4 days, and the cell density reaches 1~2×10 6 cells / ml; subculture at a passage ratio of 1:3, and continue to shake the flask at 37°C until the cell density reaches 1-2×10 6 cells / ml to obtain the seed solution; the rotation speed of the shaker flask was 150 rpm.
[0062] 3) Eight factor expression cell reactor culture
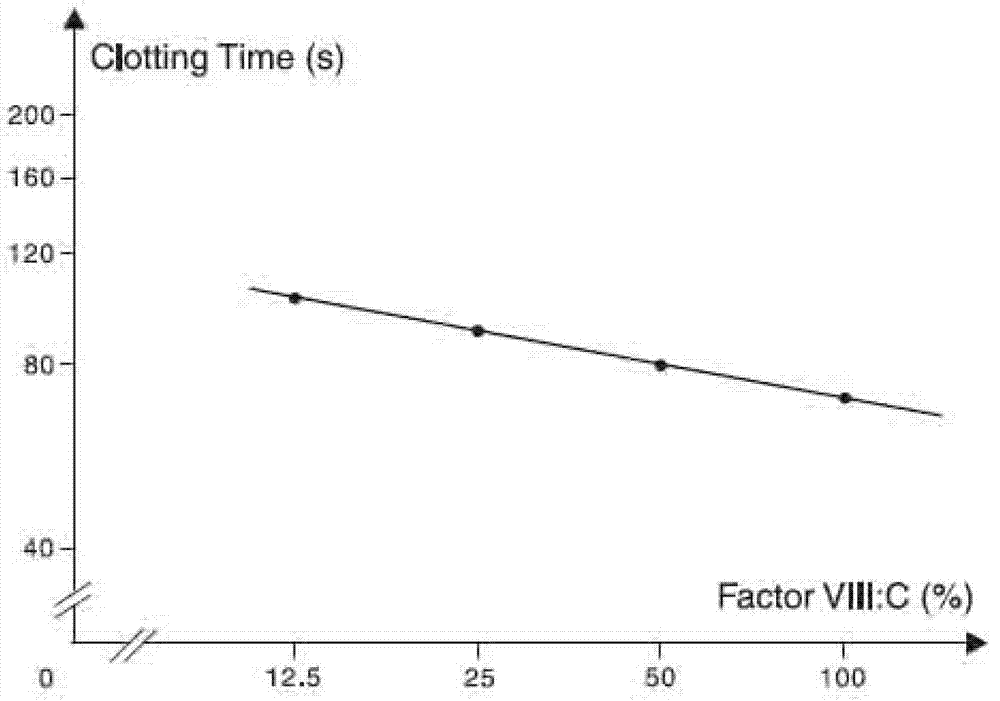
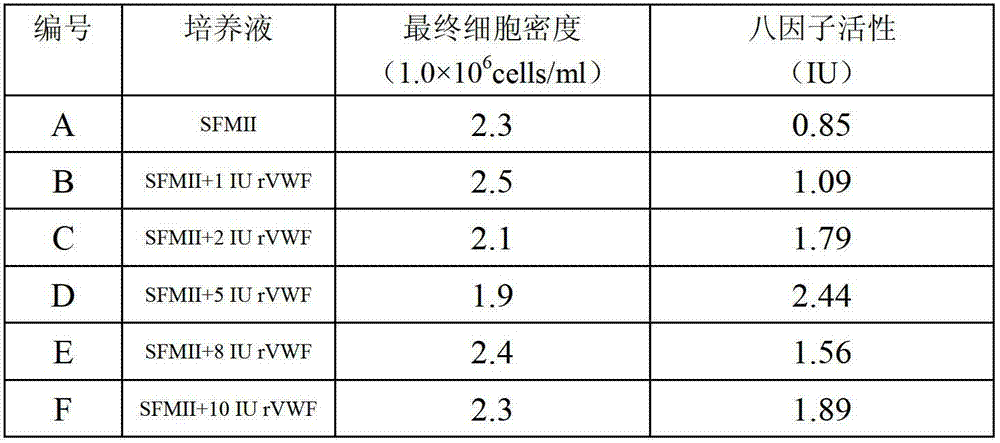
[0063] After inoculating the seed solution in the serum-free medium of the cell reactor with a 10% inoculation amount, culture it in a tank until the cell density is about 2×10 6 cells / ml, then feed-culture the cell liquid; in the feed-culture stage, the activity of the eight-factor i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com