Cell protectants comprising placenta extracts
A protective agent and extract technology, applied in the field of cytoprotective agents, can solve the problems of incomplete performance, low stem cell viability, easy loss of stem cells, etc., and achieve the effect of improving cell proliferation rate and stem cell ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0046] Preparation Example 1: Preparation of Cell Protectant
[0047] The cell protectant of the present invention was prepared by mixing amino acids listed in Table 1, minerals listed in Table 2, vitamins listed in Table 3 and other components listed in Table 4, and named "CeLLGUARD". As a control, Invitrogen's DMEM / F12 (1:1) medium further containing 10% fetal bovine serum was used for adipose-derived mesenchymal stem cells, and Lonza's KGM medium was used for keratinocytes.
[0048] [Table 1] Amino Acids
[0049]
[0050] [Table 2] Minerals
[0051]
[0052]
[0053] [Table 3] Vitamins
[0054]
[0055]
[0056] [Table 4] Other components
[0057]
Embodiment 1
[0058] Example 1: Comparison of adipocyte viability
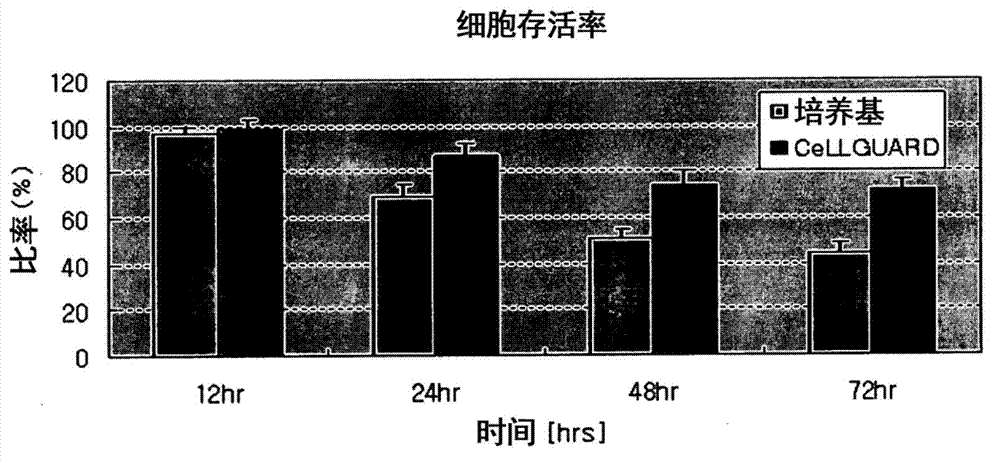
[0059] 1 x 10 isolated from adipose tissue 6 adipocytes were placed into vials containing the control medium and the cytoprotectant of the present invention (hereinafter referred to as "CeLLGUARD"). After standing at 4°C, the number of living cells was counted at predetermined time intervals to measure the viability of the cells. Cell counts were performed under a microscope using a hemocytometer after staining the cells with trypan blue.
[0060] figure 1 The cell viability of adipocytes isolated from adipose tissue stored at low temperature (4 °C) for 72 h in control medium or in CeLLGUARD solution without culture was compared. Cell viability was measured by counting viable cells. Such as figure 1 It was seen that the cell survival rate of adipocytes stored in CeLLGUARD was 72%, while the cell survival rate of cells stored in adipocyte medium was only 45%. That is, compared to the control medium, CeLLGUARD increas...
Embodiment 2
[0061] Example 2: Confirmation of stem cell ability of adipose-derived mesenchymal stem cells
[0062] 1) CFU analysis to confirm stem cell capacity
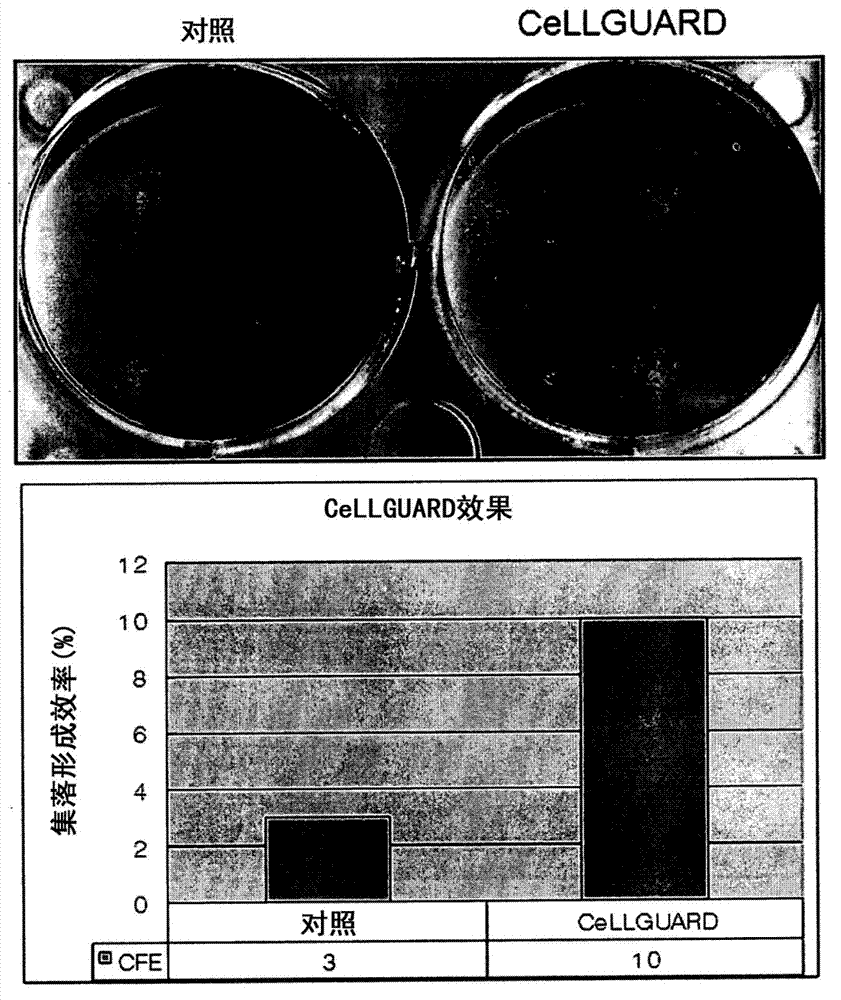
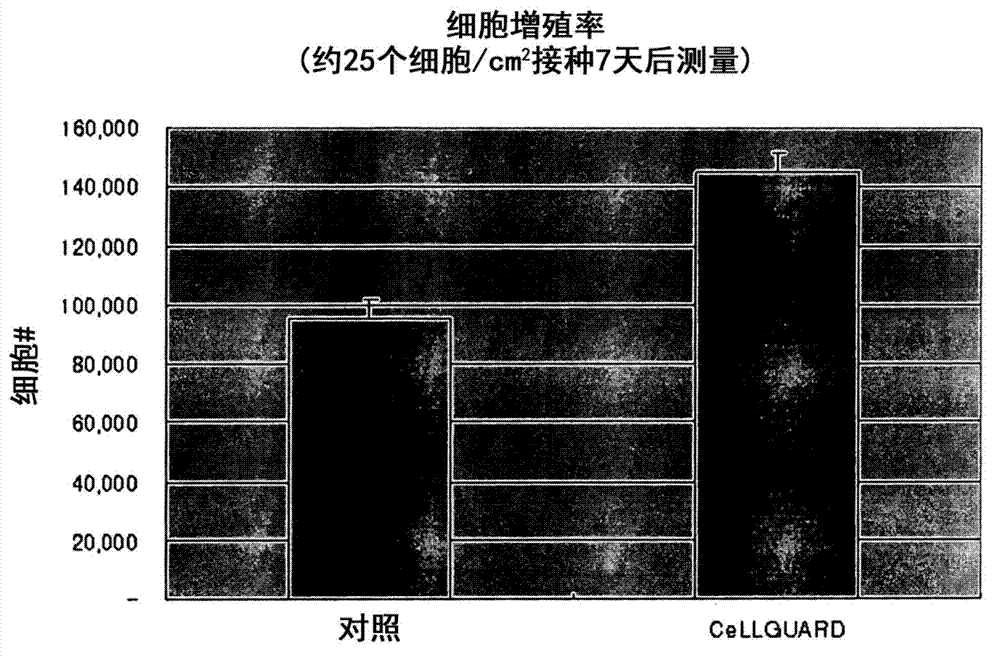
[0063] Mesenchymal stem cells derived from adipose tissue were treated with control medium or CeLLGUARD for 1 hour, and 500 cells were seeded on a 6-well plate and cultured for 7 days. To measure colony-forming units (CFU, colony-forming units), the culture medium was completely removed from the plate, and a 1:1 mixture solution of rhodan red and Nile blue was added to stain the cells for 30 minutes, and then the number of colonies was counted.
[0064] Figure 2a The stem cell capacity of adipose tissue-derived mesenchymal stem cells was compared. Adipose tissue-derived cells were treated with control medium or CeLLGUARD for 1 h, and then stem cell capacity was determined by measuring CFU. Such as Figure 2a It can be seen that CeLLGUARD obtained a colony formation efficiency (CFE) of 10%, which is more than 3 times that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com