Hepatitis B virus (HBV) specific human leukocyte antigen-A33 (HLA-A33) restrictive epitope peptides and application thereof
A hepatitis B virus, hepatitis B technology, applied in the field of immunology, can solve the problems of disappearance, decreased T cells and cellular immunity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Construction of HLA_A33-restricted epitope peptide screening platform
[0038] 1: Construction of HLA_A3303 stable cell line:
[0039] In this example, the VSVG pseudovirus system (purchased from Biovector Science Lab, Inc) was used to add NheI and XhoI sites to both ends of the human HLA_A3303 gene (hereinafter referred to as human HLA_A3303, GenBank: U09740.1). , the sequence was fully synthesized in Shanghai Xuguan Biological Company (the nucleic acid sequence is SEQ ID No: 1, and its expression can produce the protein represented by SEQ ID No: 2), and then constructed in the vector plentilox using NheI and XhoI two sites 3.7-RRP (which is the plasmid in the above-mentioned VSVG pseudovirus system (purchased from Biovector Science Lab, Inc)) forms the recombinant plasmid plentilox-HLA_A33, extracts 15 μg of the recombinant plasmid, and then mixes it with 5 μg VSVG plasmid and 5 μg RSV / REV plasmid, 5 μg of pMDLG plasmids (the above three plasmids are also ...
Embodiment 2
[0046] Example 2: Screening of HBV-specific HLA_A33-specific epitopes
[0047] Using the following resources that have been published on the Internet, the possible candidate polypeptides of HBV subtypes B and C were predicted:
[0048] Three-dimensional quantitative structure-activity relationship program is MHCPred
[0049] The scoring matrix program is SYFPEITHI
[0050] The TAP program is TAPpred
[0051] According to the results of the three, 16 peptides were selected as candidate polypeptides. The overall results are shown in Table 1 below.
[0052]
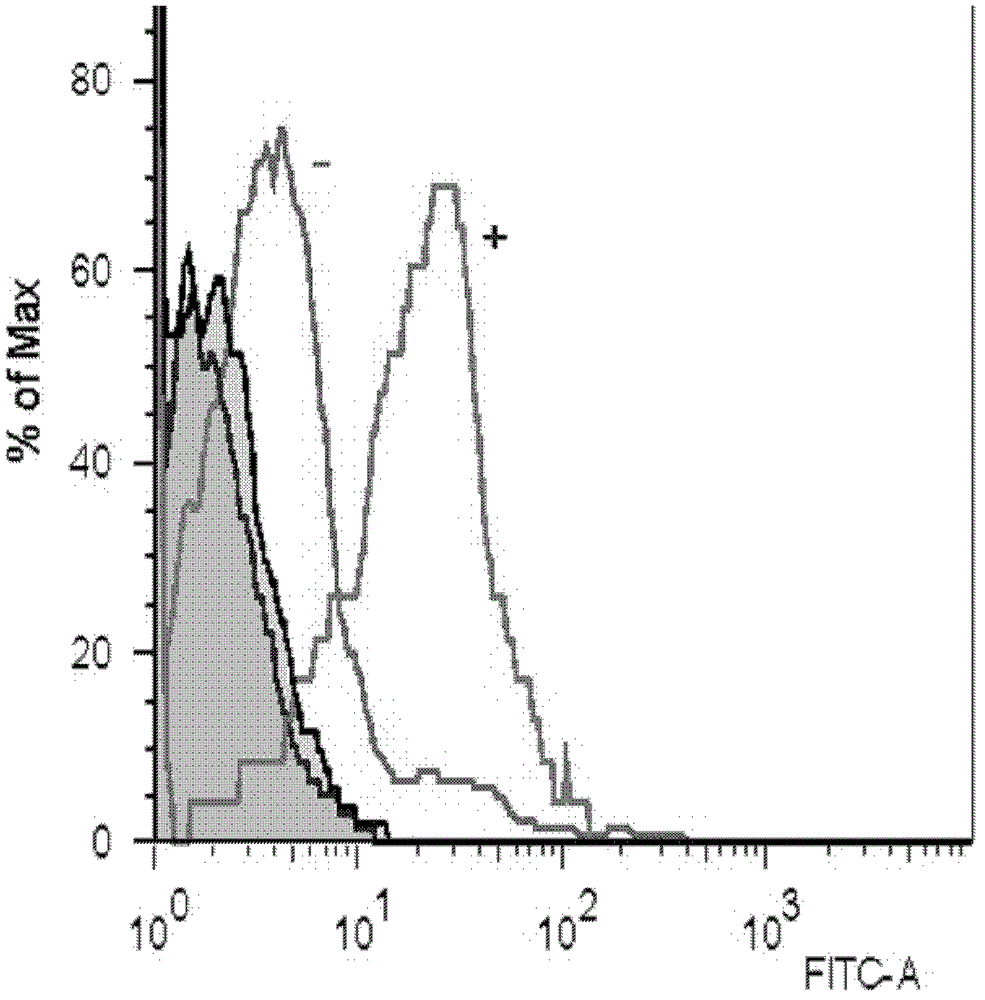
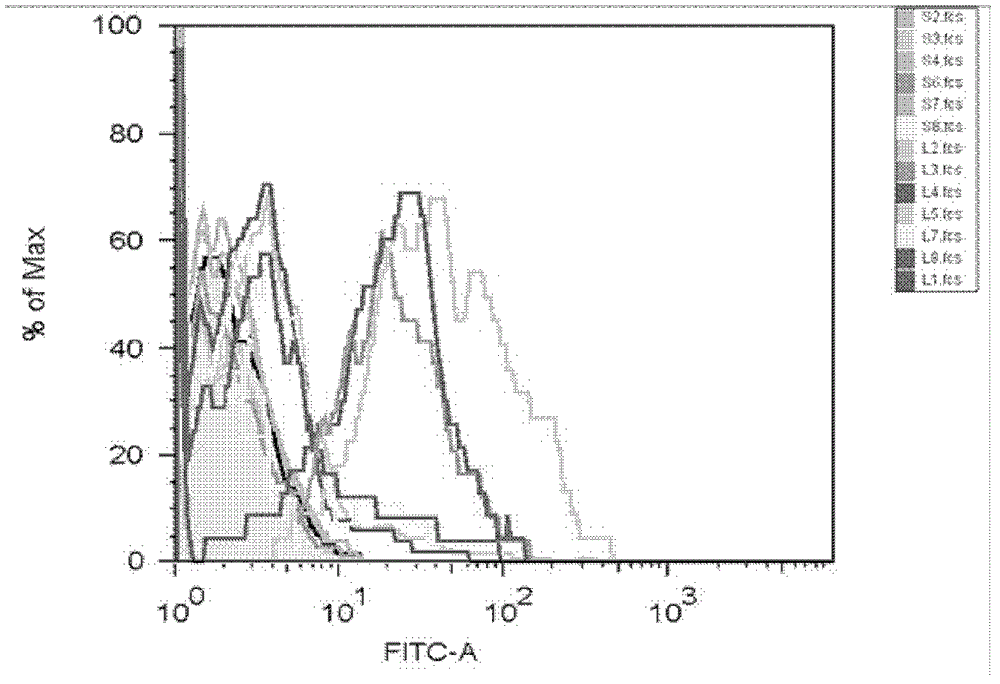
[0053] According to the method of the above-mentioned peptide binding experiment, except that the peptide concentration at the time of screening was fixed at a final concentration of 50 μM, other methods remained unchanged, and the epitope peptides that could bind to HLA_A3303 among the predicted candidate peptides were screened.
[0054] figure 2 The cell screening results of peptides 1-5 and 7-8 of the present inv...
Embodiment 3
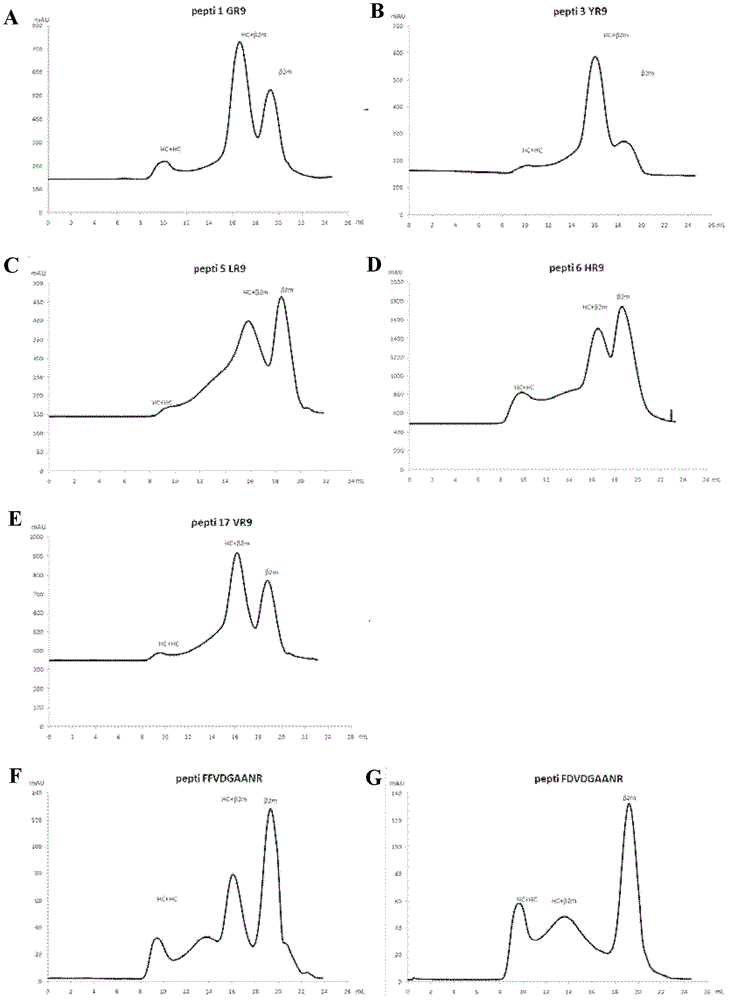
[0055] Example 3: Identification of candidate peptides by trimer renaturation
[0056] 1: HLA_A3303 Escherichia coli inclusion body expression and purification:
[0057] After optimizing the codon sequence and mRNA sequence of HLA_A3303 (the optimized coding nucleic acid sequence is SEQ ID No: 3), the optimized expression cassette was cloned into the vector pET30A (purchased from Novagen) with enzymes NdeI-XhoI to form The clone named pET30-HLA_A33HC-LND, its expression can produce the protein represented by SEQ ID No: 4, which contains α1, α2, α3 domains of HLA_A molecules and a biotinylation site sequence LND.
[0058] According to the conventional expression method, when the OD value of the Escherichia coli (E.coli) bacterial liquid reaches 0.7, add IPTG to make the final concentration 0.5mMol / L, harvest the bacteria after induction at 37°C for 4 hours, and use lysozyme (sigma) 5mg / L (per liter of original medium) treated at room temperature for 1 hour, then freeze-thawed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com