Catalytic reforming method for naphtha
A technology for catalytic reforming and naphtha, which is applied in the catalytic reforming of naphtha, processing naphtha, reforming naphtha, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] In this example, naphtha is hydrorefined.
[0047] In a 20 ml fixed bed continuous flow reactor, fill 20 ml of hydrorefining catalyst A, which contains 0.03 mass % of CoO, 2.0 mass % of NiO, 19.0 mass % of WO 3 , 0.7% by mass of F and 78.27% by mass of Al 2 o 3 . At 290°C, the hydrogen partial pressure is 1.6MPa, the hydrogen / hydrocarbon volume ratio is 200:1, and the feed volume space velocity is 8.0h -1 The naphtha with the composition and properties listed in Table 1 is hydrorefined under certain conditions. The reaction product enters the water cooler and is separated into two phases of gas and liquid, which are measured separately and analyzed for composition. The composition and properties of the refined naphtha are shown in Table 2.
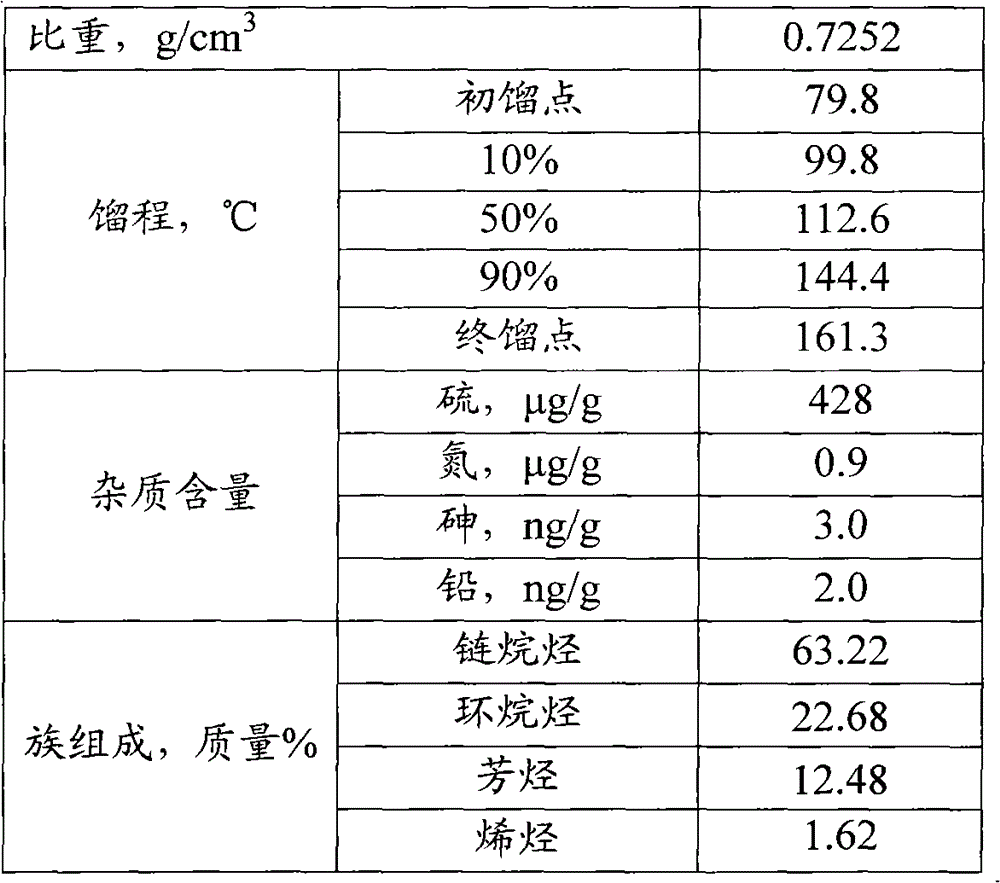
[0048] Table 1
[0049]
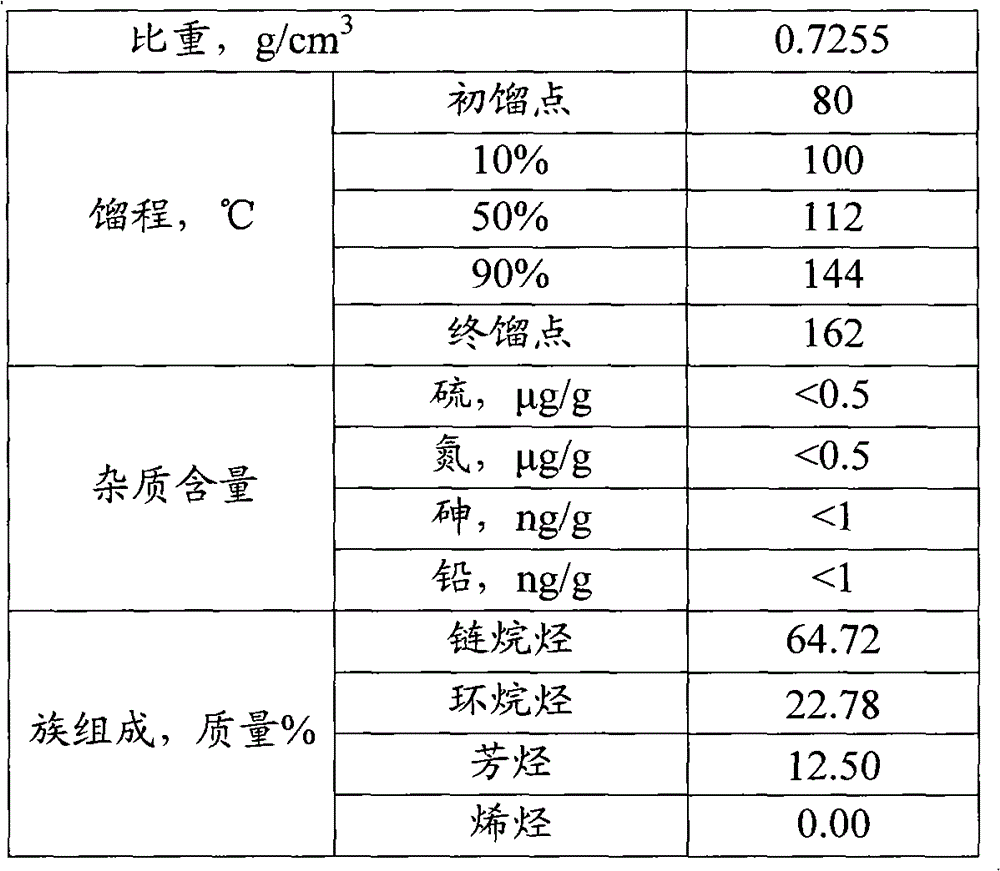
[0050] Table 2
[0051]
[0052] It can be seen from the results in Table 2 that after hydrofining, the contents of olefins, sulfur, nitrogen, arsenic and lead in naphtha meet the requirements ...
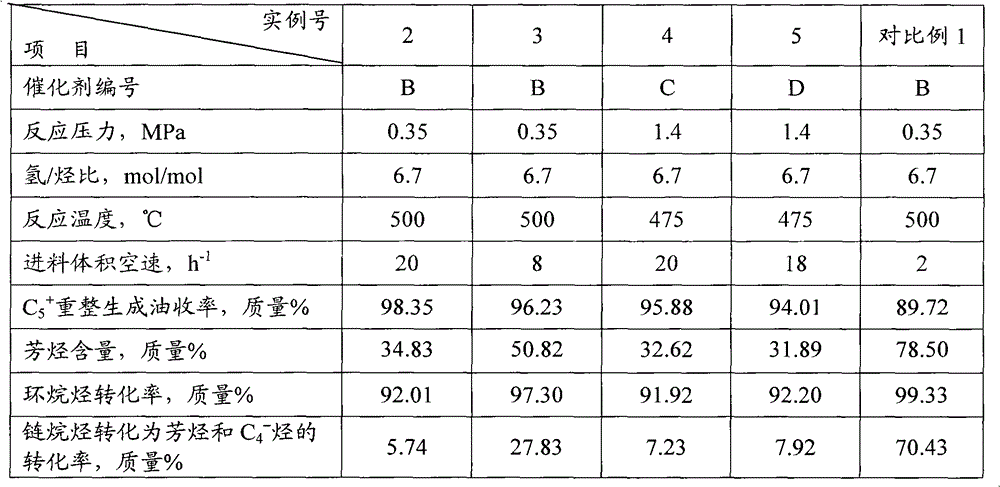
example 2~3
[0054] According to the method of the invention, the refined naphtha is catalytically reformed.
[0055] Catalytic reforming reaction using PtSn / γ-Al 2 o 3 Catalyst B, which contains 0.35% by mass of Pt, 0.30% by mass of Sn, 1.0% by mass of Cl, and the balance is γ-Al 2 o 3 .
[0056] In a 100 milliliter fixed-bed continuous flow reactor, 50 milliliters of catalyst B was loaded, and the refined naphtha listed in Table 2 was used as raw material to carry out catalytic reforming of naphtha. When the reaction raw material inlet temperature is 500°C, the reaction pressure is 0.34MPa, the hydrogen / hydrocarbon molar ratio is 6.7, and the feed volume space velocity is 20.0 and 8.0h respectively -1 The reforming reaction is carried out under certain conditions, and the reforming reaction product is rectified to obtain C 5 + Reforming produces oil, and the reaction results are shown in Table 3.
example 4
[0058] According to the method of the invention, the refined naphtha is catalytically reformed. Using PtRe / γ-Al 2 o 3 Catalyst C, which contains 0.26% by mass of Pt, 0.26% by mass of Re, 1.0% by mass of Cl, and the balance is γ-Al 2 o 3 . Before catalyst C is used, 0.1% by mass hydrogen sulfide needs to be added into the hydrogen flow at 425°C for presulfurization, so that the sulfur content of the catalyst is 0.06% by mass.
[0059]The refined naphtha listed in Table 2 is used as the raw material for catalytic reforming, when the reaction raw material inlet temperature is 475°C, the reaction pressure is 1.4MPa, the hydrogen / hydrocarbon molar ratio is 6.7, and the feed volume space velocity is 20.0h -1 The reforming reaction is carried out under certain conditions, and the reforming reaction product is rectified to obtain C 5 + Reforming produces oil, and the reaction results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com