Preparation method of alpha-cyclopentadecanone
A kind of cyclopentadecanone, hydroxyl technology, applied in the field of preparing α-hydroxy cyclopentadecanone, can solve the problems of difficult separation, influence of cyclopentadecanone purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
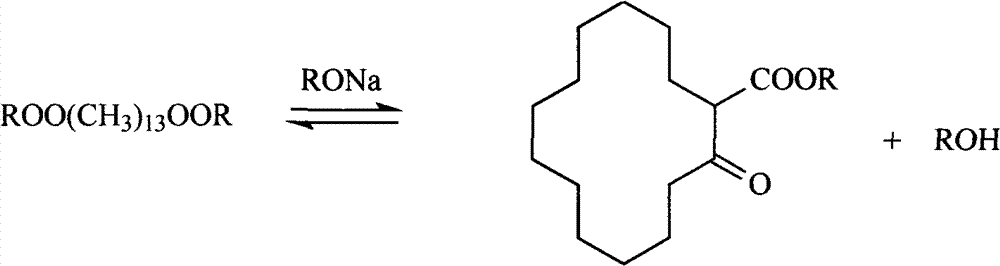
[0029] Add 11.52 g (0.03 mol) of dibutyl pentadecanedioate to an Erlenmeyer flask containing 30 ml of toluene, and mix well. Add 150ml of toluene and 20g of chlorobenzene into a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, a condenser, a dropping port, and a nitrogen port. Access to N 2 1. Heat up, when the temperature reaches 100°C, add 3.6g of metallic sodium to the flask, stir vigorously for 5min, and beat the sodium into fine sodium sand. Keeping the temperature at 100°C, a toluene solution of dibutyl pentadecanedioic acid was added dropwise, and the drop was completed in 3 hours. After the dropwise addition was completed, the reaction was kept for 1 hour. After the reaction was completed, 20ml of acetic acid was added dropwise to decompose the remaining metallic sodium. Cool down, filter, wash the filtrate with hot water to neutrality, and distill toluene under reduced pressure to obtain 7.2g. The reaction product was analyzed by gas chromat...
Embodiment 2
[0032] Add 30 g (0.1 mol) dimethyl pentadecanedioate to a Erlenmeyer flask containing 100 ml of toluene, and mix well. Add 328ml of toluene and 76g of bromopentane into a 1000ml four-neck flask equipped with a mechanical stirrer, a thermometer, a condenser, a dropping port, and a nitrogen port. Access to N 2 1. Heat up, when the temperature reaches 105°C, add 10.6g of metallic sodium to the flask, stir vigorously for 5min, and beat the sodium into fine sodium sand. Keeping the temperature at 105°C, a toluene solution of dimethyl pentadecanedioic acid was added dropwise, and the drop was completed in 4 hours. After the dropwise addition was completed, the reaction was kept for 3 hours. After the reaction was completed, the temperature was lowered, and 10 ml of methanol was added dropwise to decompose the remaining sodium metal, filtered, the filtrate was washed with hot water until neutral, and the toluene was distilled off under reduced pressure to obtain 24.2 g. The reactio...
Embodiment 3
[0035] Add 9.84 g (0.03 mol) diethyl pentadecanedioate to a Erlenmeyer flask containing 30 ml of toluene, and mix well. Add 150ml of toluene and 29g of iodobutane into a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, a condenser, a dropping port, and a nitrogen port. Access to N 2 1. Heat up, when the temperature reaches 100°C, add 3.6g of metallic sodium to the flask, stir vigorously for 5min, and beat the sodium into fine sodium sand. Keeping the temperature at 100°C, a toluene solution of diethyl pentadecanedioic acid was added dropwise, and the drop was completed in 3 hours. After the dropwise addition was completed, the reaction was kept for 2 hours. After the reaction was completed, 20ml of acetic acid was added dropwise to decompose the remaining metallic sodium. Cool down, filter, wash the filtrate with hot water to neutrality, and distill toluene under reduced pressure to obtain 6.8g. The reaction product was analyzed by gas chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com