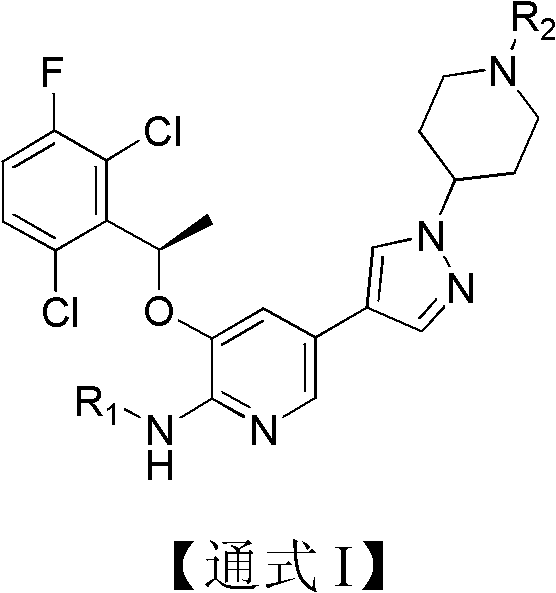
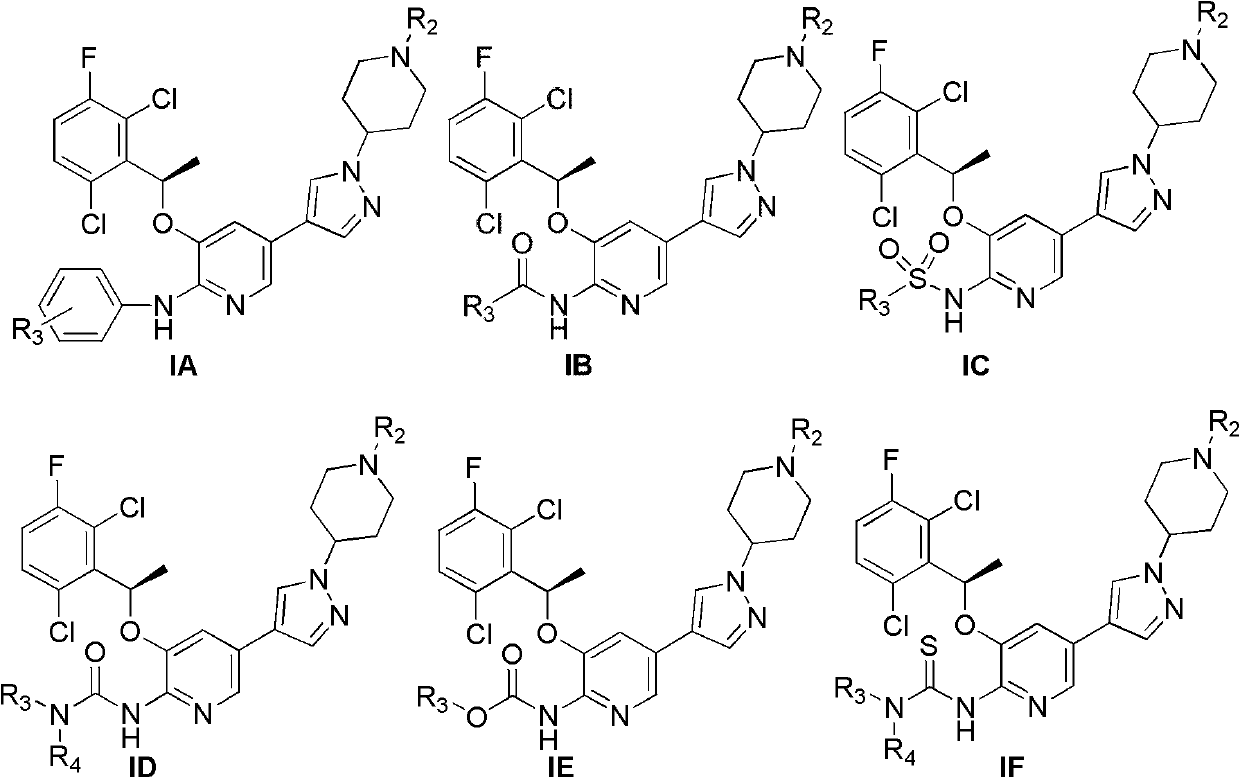
Pyridine chemical, its preparation method, and pharmaceutical composition containing the chemical and application thereof
A kind of compound and pyridine technology, applied in the field of pyridine compound, its preparation, pharmaceutical composition containing the compound and its use, can solve problems such as limited structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
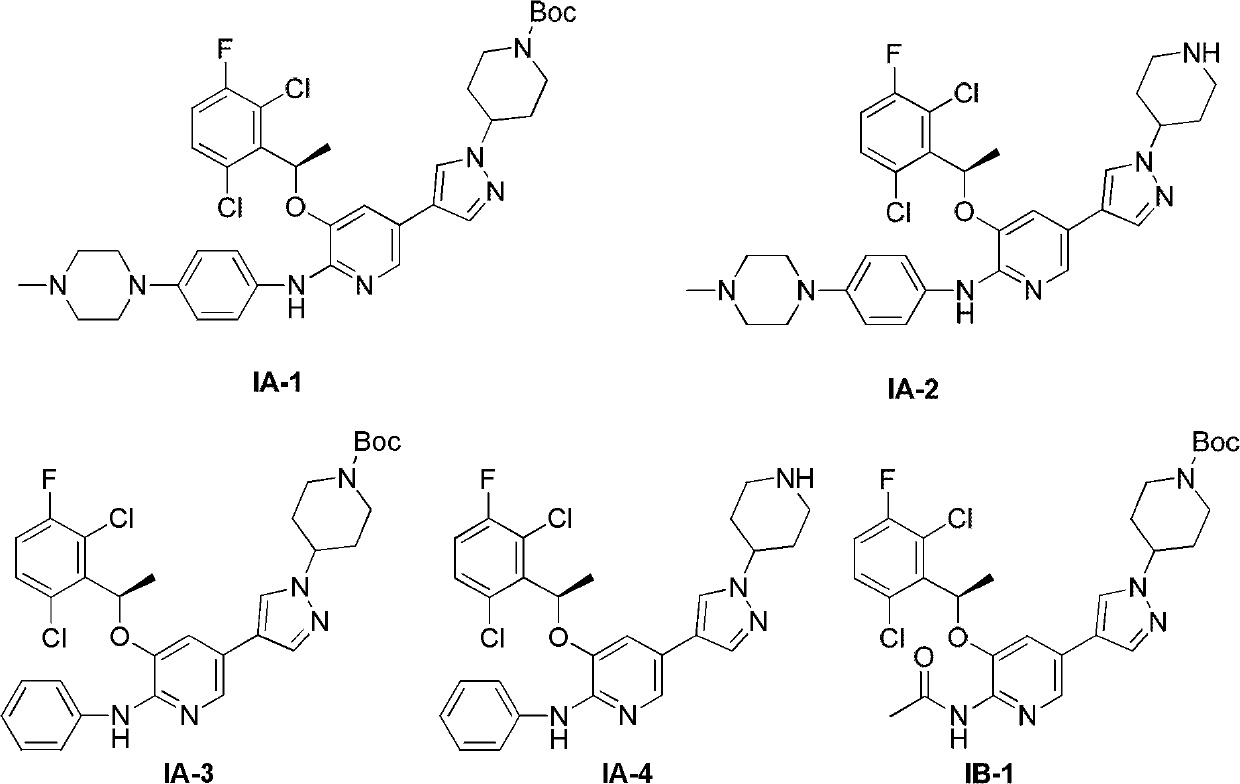
[0051] Preparation of Example 1 Compound IA-1
[0052]
[0053] Compound 1 (109.8mg, 0.2mmol), 1-(4-bromophenyl)-4-methylpiperazine (50.8mg, 0.2mmol), palladium acetate (0.015mmol), bis(diphenylphosphino) Ferrocene (0.032 mmol), cesium carbonate (106 mg, 0.28 mmol) were added to a dry round bottom flask. Evacuate and replace with nitrogen three times. 1,4-Dioxane was added under nitrogen protection. Heat to reflux and stir overnight. After filtration, the filtrate was concentrated, mixed with silica gel, and subjected to direct column chromatography (chloroform:methanol=10:1) to obtain compound IA-1 (110 mg). 1 H NMR (300MHz, CDCl 3 )δ7.88(s, 1H), 7.56(m, 3H), 7.49(s, 1H), 7.30(m, 3H), 7.10(s, 1H), 7.05(t, J=7.5Hz, 1H), 7.03(s, 1H), 6.93(s, 2H), 6.14(q, J=6.6Hz, 1H), 4.23(m, 3H), 3.17(s, 4H), 2.90(m, 2H), 2.60(m , 4H), 2.38(s, 3H), 2.16(m, 2H), 1.94(m, 2H), 1.89(d, J=6.6Hz, 3H), 1.48(s, 9H).
preparation Embodiment 2
[0054] Preparation of Example 2 Compound IA-2
[0055]
[0056] Trifluoroacetic acid (0.1 mL) was added dropwise to a dichloromethane solution (5 mL) of compound IA-1 (100 mg), stirred at room temperature overnight, and concentrated. Add ammonia in methanol (10% X 20mL) solution to the concentrated solution, and concentrate. The sample was mixed with silica gel and subjected to direct column chromatography (chloroform:methanol=10:1) to obtain compound IA-2 (75 mg). 1 H-NMR (300MHz, CDCl 3 ):δ 1 H NMR (300MHz, CDCl 3 )δ7.67(s, 1H), 7.44(m, 2H), 7.34(m, 2H), 7.20(m, 1H), 6.94(m, 3H), 6.14(q, J=6.6Hz, 1H), 4.23(m, 3H), 3.17(s, 4H), 2.90(m, 2H), 2.60(m, 4H), 2.38(s, 3H), 2.16(m, 2H), 1.94(m, 2H), 1.89 (d, J=6.6Hz, 3H).
preparation Embodiment 3
[0057] Preparation of Example 3 Compound IA-3
[0058]
[0059] Compound IA-3 was synthesized in the same manner as compound IA-1 of Preparation Example 1, except that bromobenzene was used instead of 1-(4-bromophenyl)-4-methylpiperazine. 1 H NMR (300MHz, CDCl 3 )δ7.92(s, 1H), 7.70(m, 2H), 7.61(s, 1H), 7.51(s, 1H), 7.31(m, 3H), 7.05(t, J=8.1Hz, 1H), 6.98(m, 2H), 6.16(q, J=6.6Hz, 1H), 4.25(m, 3H), 2.90(m, 2H), 2.14(m, 2H), 1.92(m, 5H), 1.48(s , 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com