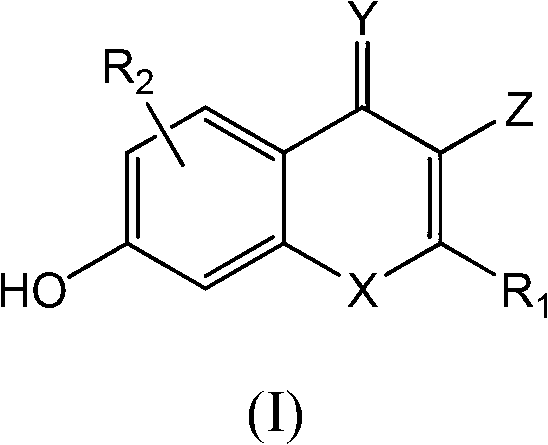
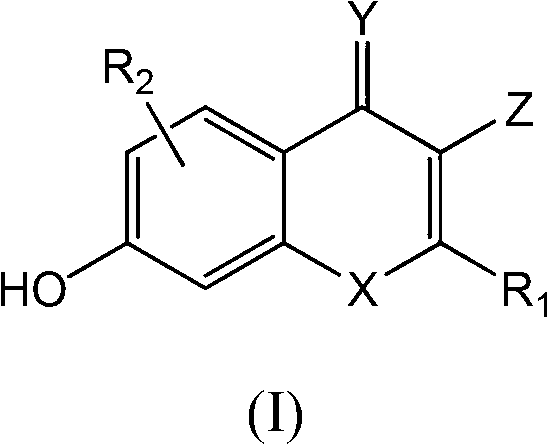
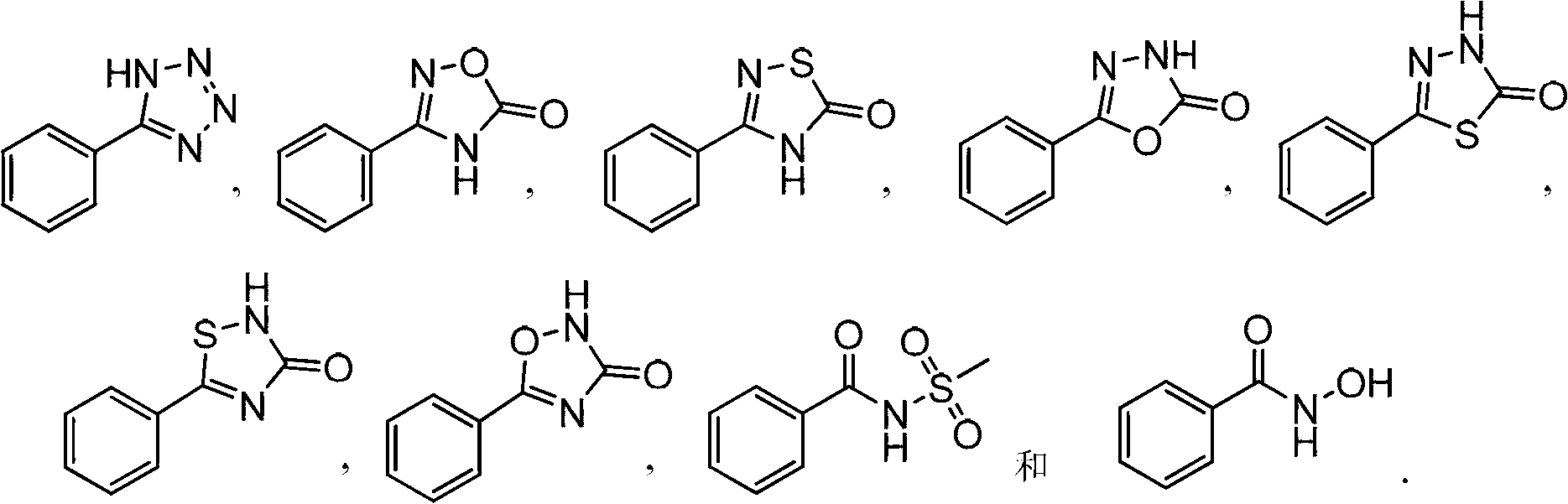
Novel S-nitrosoglutathione reductase inhibitors
A hydroxyl and alkyl technology, applied in the field of new compounds, can solve problems such as unstable reactivity and short lifetime of NO
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Example 1: 3-(4-(1H-tetrazol-5-yl)phenyl)-7-hydroxy-2-(trifluoromethyl)-4H-benzopyran-4-one
[0212]
[0213] synthesis:
[0214] Step 1: Synthesis of 4-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)benzonitrile
[0215] At 0°C, to 4-(2-(2,4-dihydroxyphenyl)-2-oxoethyl)benzamide (Intermediate A) (300mg, 1.08mmol) and triethylamine (TEA ) (0.6ml, 4.32mmol) in DCM (6ml) was added TFAA (1.2ml, 8.64mmol) dropwise. The mixture was stirred at room temperature for 2 hours. Then mix the mixture with 1N HCl solution (5ml), saturated NaHCO 3 (5ml) and brine (5ml) wash. Use Na for organic phase 2 SO 4 It was dried, concentrated, and purified by prep-TLC (PE (petroleum ether): EtOAc=3:1) to obtain a yellow oily product (66 mg, 19.5%). MS(ESI): m / z 332.1[M+1] + .
[0216] Step 2: Synthesis of 3-(4-(1H-tetrazol-5-yl)phenyl)-7-hydroxy-2-(trifluoromethyl)-4H-benzopyran-4-one
[0217] At room temperature, add 4-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)benzonitrile (5...
Embodiment 2
[0219] Example 2: 5-(7-Hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)thiophene-2-carboxylic acid
[0220]
[0221] synthesis:
[0222] Step 1: Synthesis of methyl 5-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)thiophene-2-carboxylate
[0223] Follow the method described in Step 1 of Example 1, starting with Intermediate B. MS(ESI): m / z371.0[M+1] + .
[0224] Step 2: Synthesis of 5-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)thiophene-2-carboxylic acid
[0225] To 5-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)thiophene-2-carboxylic acid methyl ester (295mg, 0.80mmol) in dioxane Concentrated HCl (1.5ml) was added to the solution in alkane (1.5ml). The reaction mixture was stirred at 70°C for 24 hours, cooled to room temperature and centrifuged. The precipitate was rinsed with water (2ml×2), DCM (2ml×2), and dried in vacuum to obtain the desired product of Example 2 (216.1 mg, 76.3%) as a gray powder.
[0226] data: 1 H NMR(MeOD-d 4 500MHz TMS)...
Embodiment 3
[0227] Example 3: (trans)-4-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)cyclohexanecarboxylic acid
[0228]
[0229] synthesis:
[0230] Step 1: Synthesis of ethyl 4-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)cyclohexanecarboxylate
[0231] The method of step 1 of Example 1 was followed, starting with Intermediate C, where the crude product was directly applied without post-treatment or purification. MS(ESI): m / z 385.1[M+1] + .
[0232] Step 2: To 4-(7-hydroxy-4-oxo-2-(trifluoromethyl)-4H-benzopyran-3-yl)cyclohexane carboxyacetate (450 mg, 1.1 mmol) The solution in dioxane (3ml) was added with concentrated HCl (3ml). The solution was stirred at 75°C overnight. The mixture was concentrated in vacuo to obtain a yellow solid, which was purified by prep-HPLC to obtain the pure trans isomer of the desired product of Example 3 (100 mg, 24%).
[0233] data: 1 H NMR(MeOH-d4 500MHz TMS): δ7.97(d,J=9.0Hz,2H), 6.97(dd,J=2.0,8.5Hz,1H), 6.84(d,J=2.5Hz,1H), 2.71(t,J=12.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com