New application of P53 gene
A new application, P53 protein technology, applied in gene therapy, medical preparations containing active ingredients, metabolic diseases, etc., can solve the problems of insulin deficiency, poor insulin effect, etc., to achieve short production cycle, easy control of process conditions, Simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Material
[0021] 1. Test substance: Recombinant human P53 adenovirus injection, National Medicine Standard S20040004, main ingredients: Recombinant adenovirus-P53 gene particles; Excipients: trihydroxyaminomethane, glycerol. Specification: 1×10 12 VP / support. Purchased from a commercially available recombinant human P53 adenovirus injection, produced by Shenzhen Sibio Gene Technology Co., Ltd., batch number: 20070901.
[0022] 2. Animals:
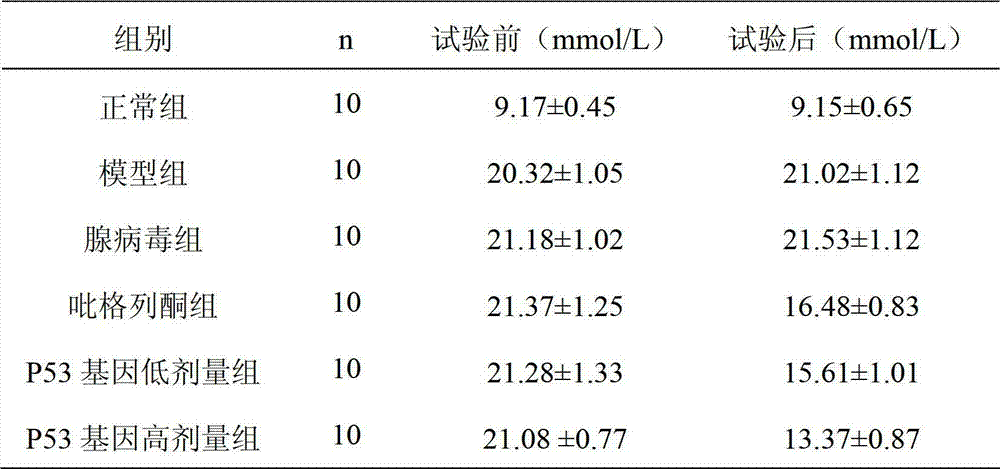
[0023] db / db mouse: Type 2 diabetes mouse model, purchased from Nanjing Pengsheng Biotechnology Development Co., Ltd., to study the hypoglycemic effect of p53 gene.
[0024] db / m mice: purchased from Nanjing Pengsheng Biotechnology Development Co., Ltd., as a control group of normal mice.
[0025] Two, method
[0026] Fifty 6-week-old db / db mice with a weight of 45.8±3.3g were randomly divided into five groups, each with 10 mice, namely the p53 gene low-dose group, p53 gene high-dose group, pioglitazone group, adenovirus group, and model ...
Embodiment 2
[0045] 1. Material
[0046] 1. Test substance: Recombinant human P53 adenovirus injection, National Medicine Standard S20040004, main ingredients: Recombinant adenovirus-P53 gene particles; Excipients: trihydroxyaminomethane, glycerol. Specification: 1×10 12 VP / support. Purchased from a commercially available recombinant human P53 adenovirus injection, produced by Shenzhen Sibio Gene Technology Co., Ltd., batch number: 20070901.
[0047] 2. Treat the crowd
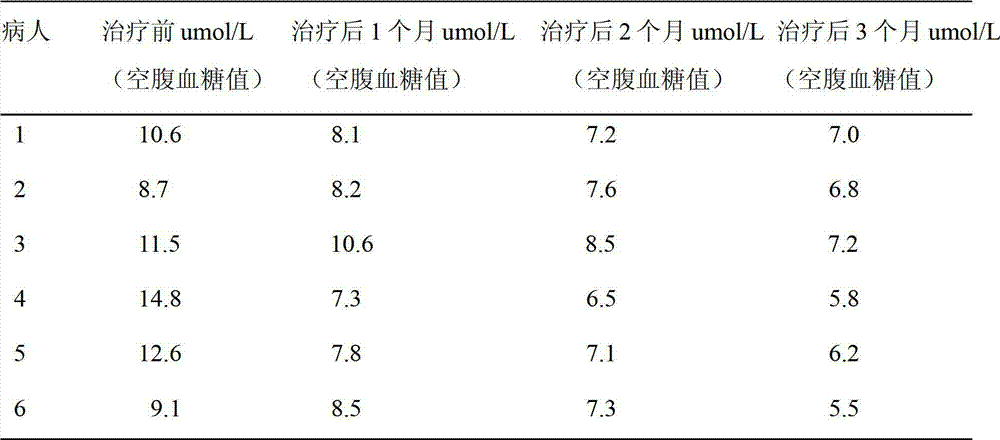
[0048] There were 6 patients with type 2 diabetes, all male, aged 43-76 years old, with an average of 56.7 years old. Fasting blood glucose was 8.9~12.3umol / L, with an average of 10.6umol / L. All patients were treated with insulin. The application time of insulin was 1 to 5 years, with an average of 2.6 years. The insulin dosage was 12u~48u, and the average daily dosage was 26u.
[0049] Before treatment, the patient signs a consent form for the use of special medications.
[0050] Treatment method: Recombinant human P53 adenoviru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com