Polypeptide for enclosing TGF-beta acceptor or IL-10 acceptor, pharmaceutical composition and application
A composition and drug technology, applied in the field of immunobiology and biomedicine, can solve the problems of virus-induced genes, difficulty in obtaining humanized antibodies, heterogeneous antibody immunogenicity, and limited clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Design and synthesis of polypeptides (short peptides)
[0055] Each short peptide is artificially synthesized at 5 mg, synthesized by Shanghai Keyeptide Biotechnology Co., Ltd., diluted to 10nM, and the final concentration is 10pM when used, and mixed in equal proportions. As shown in Table 1 below.
[0056] Table 1: Synthetic peptides (short peptides):
[0057]
[0058]
Embodiment 2
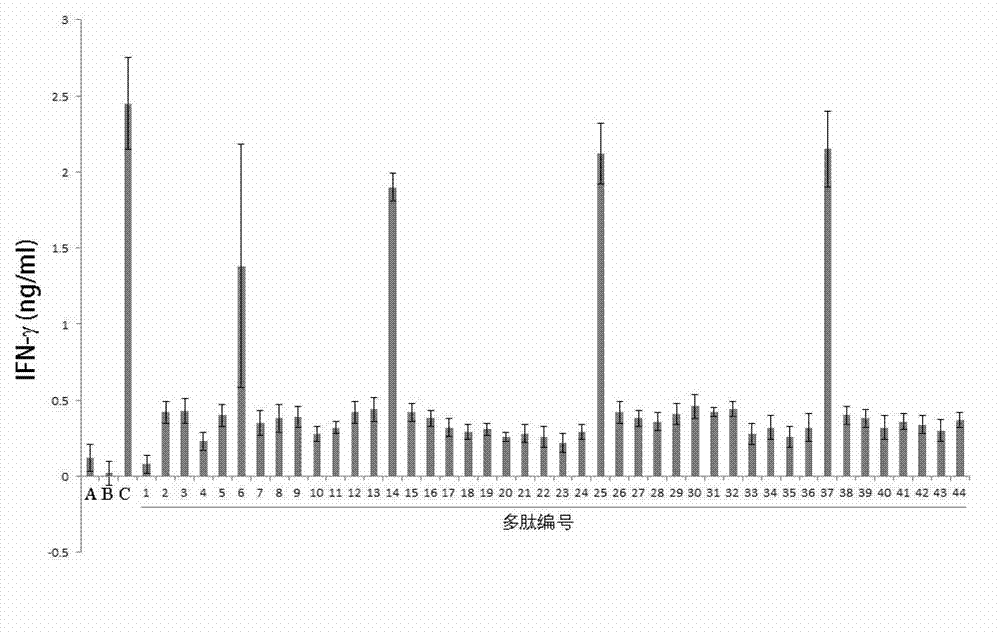
[0059] Example 2: Screening of polypeptides (short peptides) with blocking effect
[0060] Sample: 44 polypeptides in Example 1.
[0061] Screening method: After synthesizing the polypeptide sequence according to Table 1, prepare it to a concentration of 10 nM. Blood PBMCs from tumor patients were separated, and CD4CD25 and CD8 were sorted out by flow cytometry. CD4CD25 is kept for future use. CD8 was cultured in vitro, and the culture medium was a serum-free medium purchased from QIAGEN in the United States. The No. 45 polypeptide described in this example was used to activate CD8 cells for 3 days under the presentation of D1 cells (purchased from ATCC and cultured in our laboratory). At the same time, CD3 and IL-2 were used to expand the activated CD8 T cells. Cells were collected and frozen for later use. In the CD4CD25, CD8 co-culture system established in our laboratory, the activated CD8 was first added with a final concentration of 10pM blocking polypeptide to act...
Embodiment 3
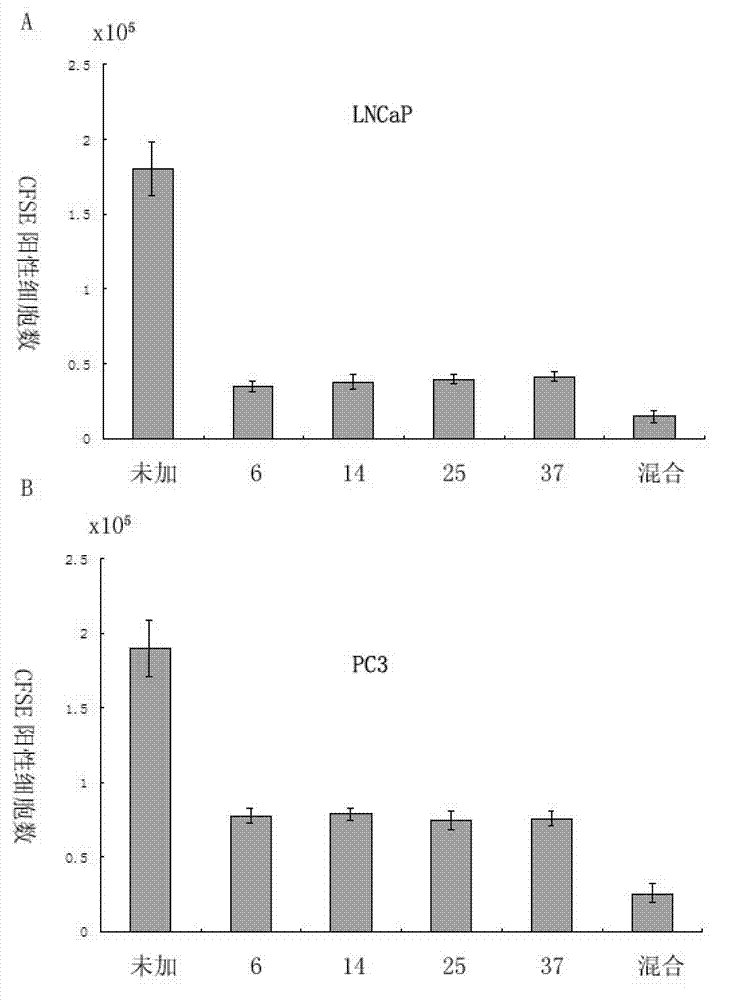
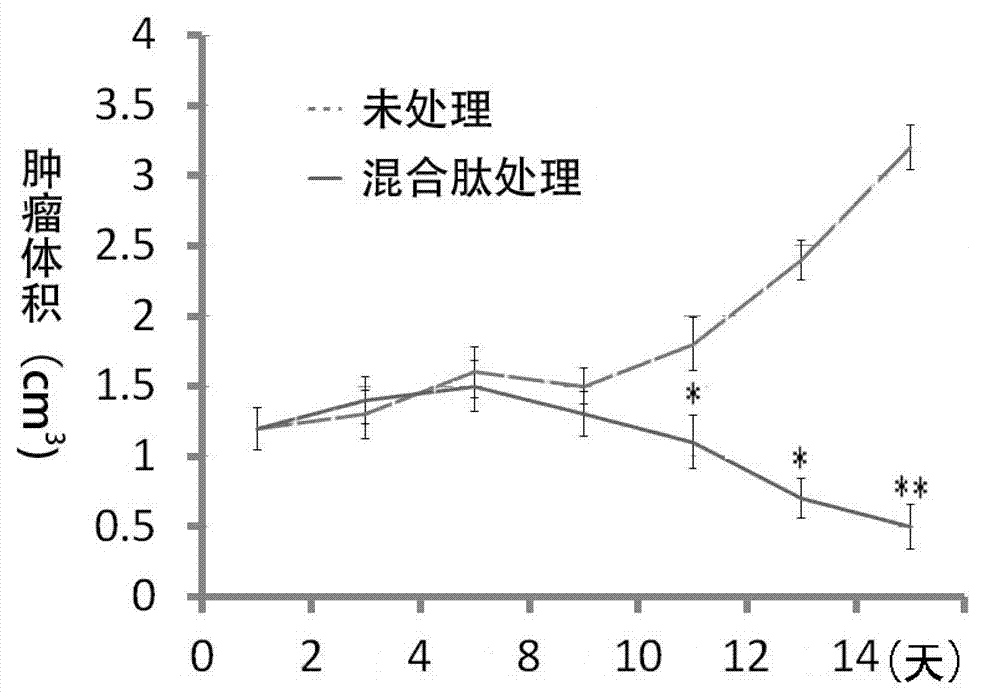
[0065] Embodiment 3: antitumor experiment of mixed polypeptide
[0066] Tumor target cells LNCaP were added to the co-culture system ( figure 2 A) or PC3 cells ( figure 2 B) (2X10 5 ), showing that the mixture of peptide 6, peptide 14, peptide 25, and peptide 37 has a particularly strong tumor-killing effect.
[0067] Method: Firstly label target cells with CFSE [Quah BJ, Parish CR. New and improved methods for measuring lymphocyte proliferation in vitro and in vivo using CFSE-like fluorescent dyes. J Immunol Methods. 2012 May 31; 379(1-2): 1-14. Epub 2012 Feb 21.], Addition of 1X10 containing peptide treated or untreated 6 activated CD8 + T cells and 1X10 5 In the three-day co-culture system of regulatory T cells, the number of CFSE-positive cells was detected by flow cytometry. The epitope peptides and mimic epitope peptides for T cell restimulation are still VAITLLSLV and VAIMLLSLV.
[0068] figure 2 A-B shows that the mixed peptides of peptides 6, 14, 25, and 37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com