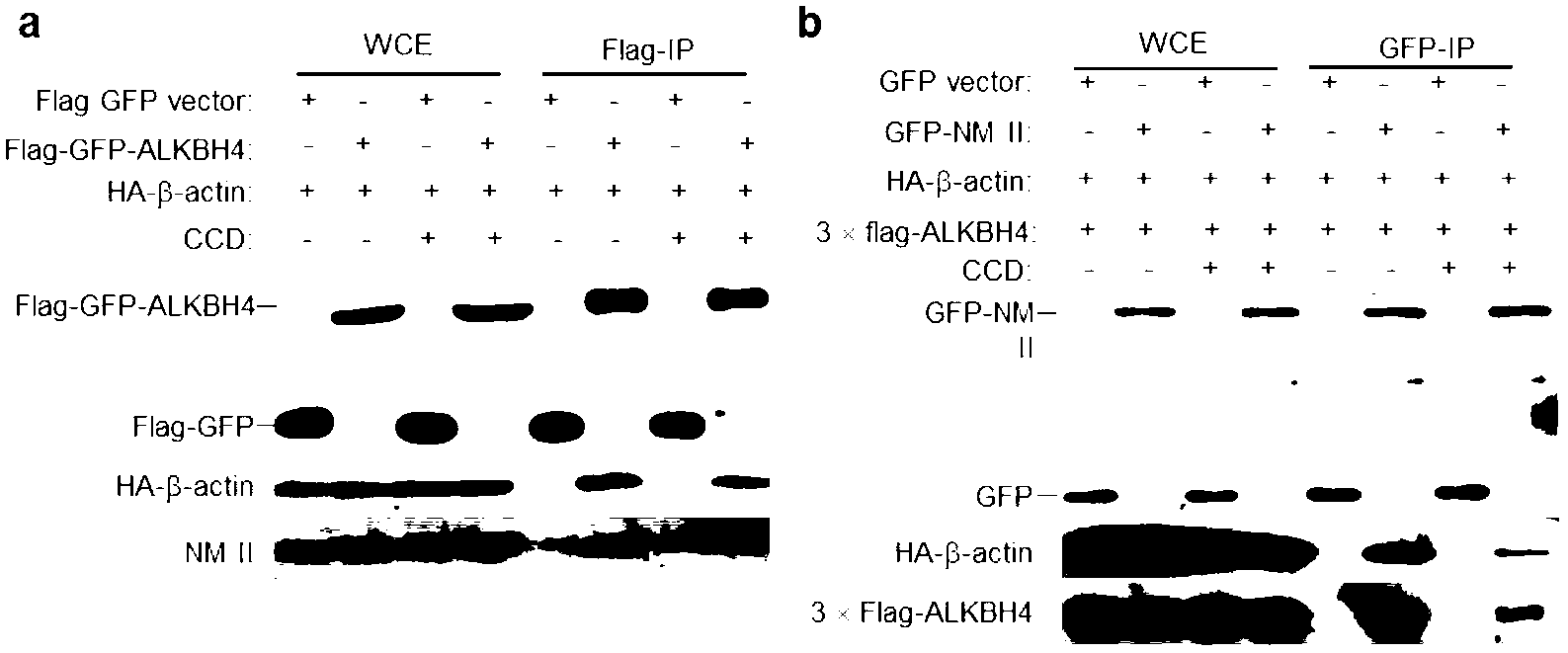
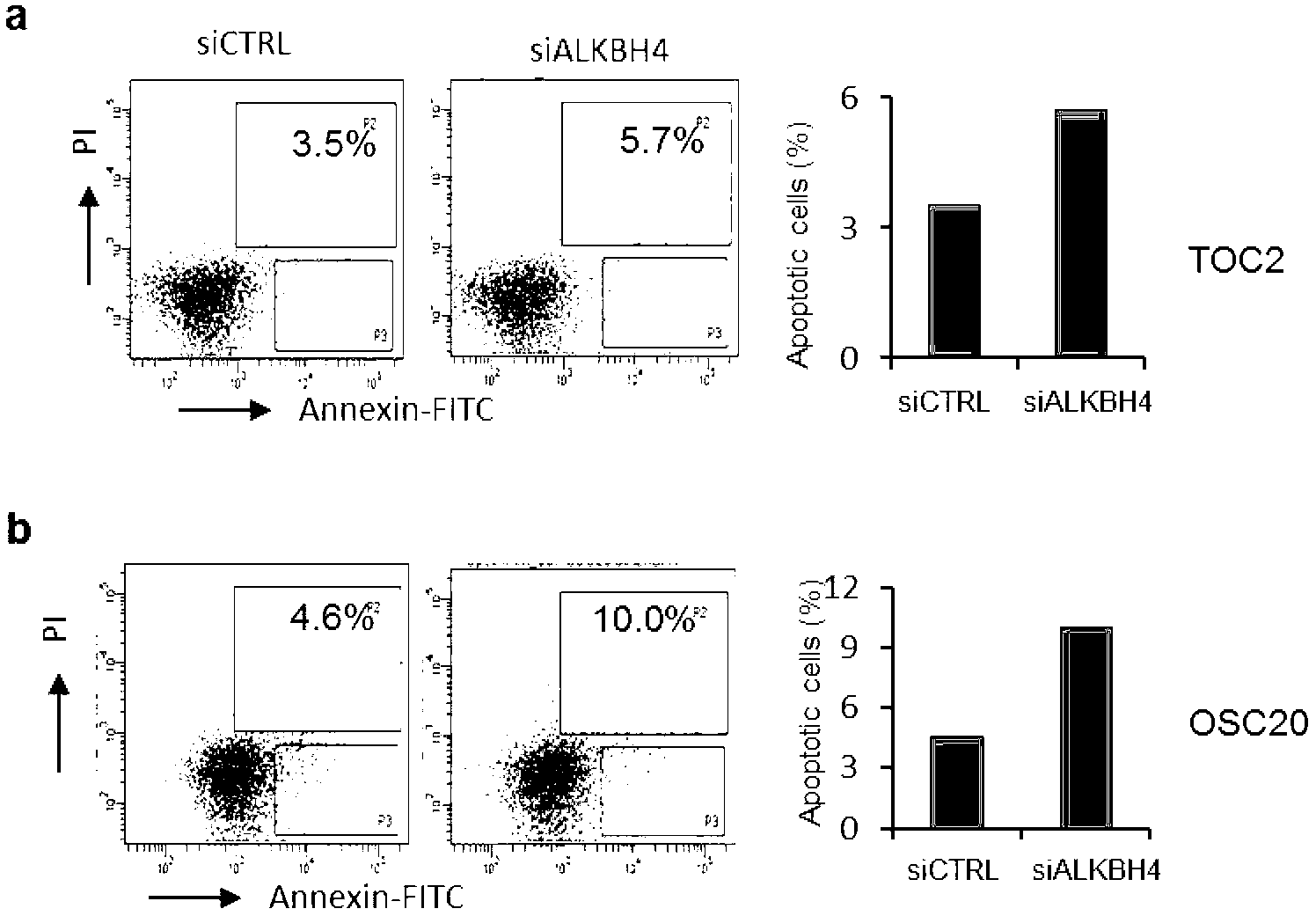
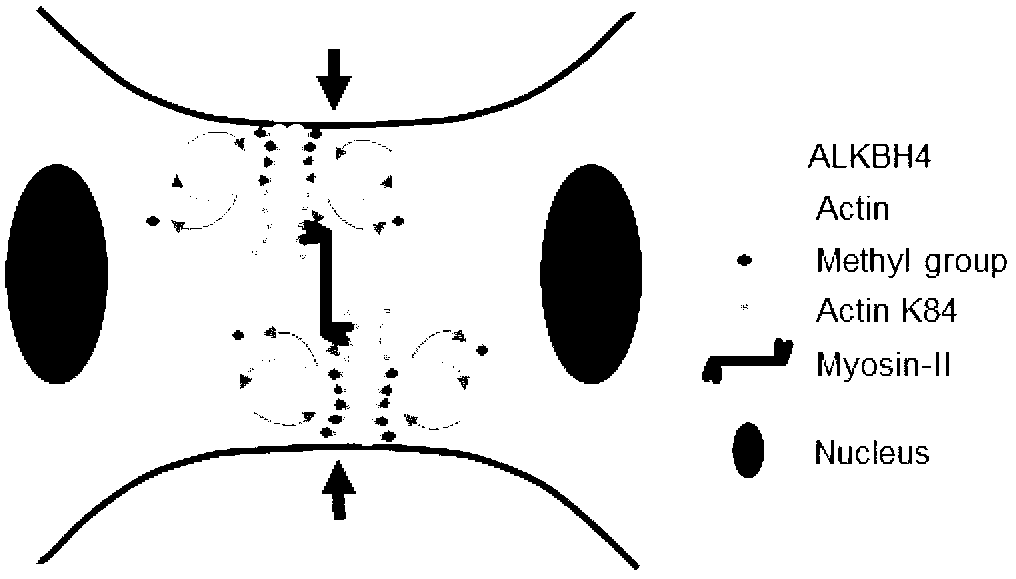
Function of actin 84 lysine monomethylation in cytokinesis and cell proliferation as well as application thereof to drug development
A technology of actin and lysine, applied in peptide/protein components, medical preparations containing active ingredients, applications, etc., can solve problems such as unclear structure and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Experimental methods and materials applied in the embodiments of the present invention
[0045] Experimental Materials:
[0046] 1. Information on the cell lines used
[0047]
[0048] 2. Antibody information used
[0049]
[0050]
[0051] 3. Plasmid information used
[0052]
[0053] 4. Chemical reagents used
[0054] Nocodazole (Sigma, M1404), Cytochalasin D (Sigma, C8273) and Blebbistatin (Sigma, B0560), taxol (Invitrogen P3456) and phalloidin (Invitrogen P3457), Lipofectamine 2000 (Invitrogen), Lipofectamine RNAiMAX (Invitrogen), PI-stained kit (Becton-Dickinson), Annexin V-FITC & PI Detection Kit (Jiamay Biotech, Beijing, China), DAPI (Vector Laboratories, Burlingame, CA), anti-flag M2 affinity gel (sigma, A2220), anti-HA affinity gel (sigma), GFP-Trap-A beads (ChromoTek, gta-20), anti-Rabbit Ig IP Beads (eBioscience), anti-Mouse Ig IP Beads (eBioscience), ECL Western Blotting Detection Kit (GE)
[0055] experimental method:
[00...
Embodiment 2
[0200] Example 2: Purification of ALKBH4 fusion protein to prepare rabbit-derived antibody against ALKBH4 protein
[0201] 1. ALKBH4 protein domain and possible activity mechanism
[0202] Escherichia coli AlkB protein as mononuclear non-heme Fe 2+ And α-ketoglutarate-dependent dioxygenase superfamily members, which use metal ions as prosthetic groups and oxygen molecules as co-substrates, activate dioxygen molecules to catalyze the oxidation of methyl groups that are in contact with nitrogen atoms on stable heterocycles group, causing a conformational change that removes the methyl group by releasing formaldehyde. ALKBH protein is a member of the human dioxygenase AlkB protein family. The commonality of this family protein is that it has the functional domain of Escherichia coli AlkB protein: the ferrous ion binding domain is HxDx n The H domain, α-ketoglutarate and the substrate binding domain, the RxxxxxR domain. The ALKBH4 protein also has such domains: iron ion binding...
Embodiment 3
[0216] Example 3: Verification of the localization and expression of the ALKBH4 gene in cells
[0217] 1. The ALKBH4 protein is localized on the centrosome.
[0218] In this experiment, immunofluorescence technique was used to verify the localization of ALKBH4 in cells. MRC5 cells were inoculated into six-well plates covered with coverslips at a seeding density of 30-40%, and treated with Nocodazole at a final concentration of 0.04 μg / ml the next night. 16 hours, the purpose of adding Nocodazole is to arrest the cells in mitosis. After 16 hours, wash once with PBS, replace with fresh complete medium to continue culturing for 1 hour, wash twice with PBS, fix and osmotically drill holes, the specific method is the same as in Example 1. The centrosome marker γ-tubulin and the spindle marker α-tubulin were hybridized with ALKBH4 antibody as the primary antibody, and the secondary antibody was hybridized with FITC-conjugated anti-mouse secondary antibody and Cy3-conjugated anti-ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com